Cutting-edge strategies set to increase our access to chemical space after researchers use them to verify unprecedented structures
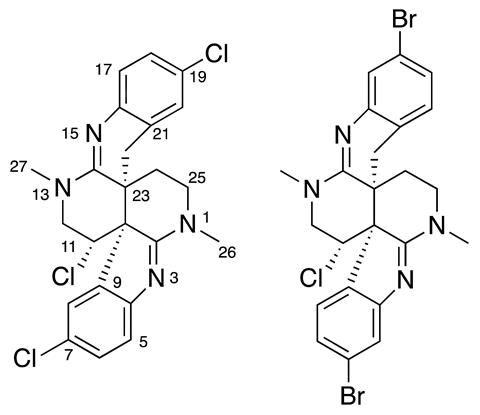
Scientists have identified the structures of two marine natural products that were previously considered too complicated to characterise.1 A combination of well-known spectroscopic tools and new experiments probing orientation-dependant bonding allowed the team to unpick the structures.
Natural products are a rich source of pharmacologically-active compounds. The problem is: they are often difficult to purify and identify.
Gary Martin, of Merck Research Laboratories in the US, and Kirk Gustafson, from the US National Cancer Institute, have been studying and characterising natural products for years. ‘There has been a continuing flow of incorrectly reported complex natural product structures into the published literature … at present, there are more than 1200 structure revision papers. Stopping investigators from reporting incorrect structures in the first place will free up their time to pursue and identify new molecular entities,’ they say.
Read the full story by Hannah Kerr on Chemistry World.
1 D J Milanowski et al, Chem. Sci., 2017, DOI: 10.1039/c7sc01996c (This paper is open access.)