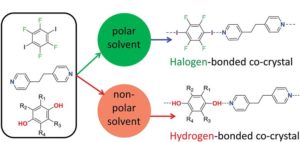
Solvent effects control competition between hydrogen bonding and halogen bonding in supramolecular systems, new research shows. The upshot of the finding is a potential new tool to direct supramolecular self-assembly.
During self-assembly, each molecule breaks its bonding interactions with neighbouring solvent molecules, then forms new interactions. To investigate competition between hydrogen bonding and halogen bonding when co-crystals form, researchers from an ongoing collaboration between the UK Universities of Sheffield, York and Cambridge chose seven solvents of different polarities to study three aromatic molecules known to self-assemble. The molecules’ functional groups included pairs of hydrogen bond and halogen bond donors that compete for a common acceptor group.
Read the full story by Fiona Tscherny on Chemistry World.