The first pentavalent praseodymium nitride–oxide that features a rare Pr≡N triple bond is the second ever lanthanide(V) complex to be made.
Lanthanide chemistry is dominated by the +3 oxidation state. There are some common +4 lanthanide complexes such as cerium oxide (CeO2), but +5 compounds have proven elusive. Praseodymium has long been considered as the most promising route to lanthanide(V) chemistry as it has five valence electrons, but the first pentavalent praseodymium complex, the oxide PrO2+, was only synthesised last year.
Source: © Royal Society of Chemistry
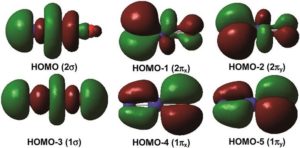
Calculations led to these representations of the molecular orbitals of the praseodymium(V) compound, NPrO
Read the full story by Aurora Walshe on Chemistry World.










