The ability to select which common building blocks of a mixture react, producing different products on demand, holds great promise for chemical synthesis. Think about systems where you have to add a great deal of additional components to prevent one reaction route and initiate another; wouldn’t it be simpler if you could add just one component that switches the chemical transformation?
This is what David Leigh and his team from the School of Chemistry at The University of Manchester have done. They have created a rotaxane with two different activation sites which promote different reactions and thus different products in the same mixture. The macrocycle position within the rotaxane is controlled and leads to one of the active sites being blocked while the other is active.
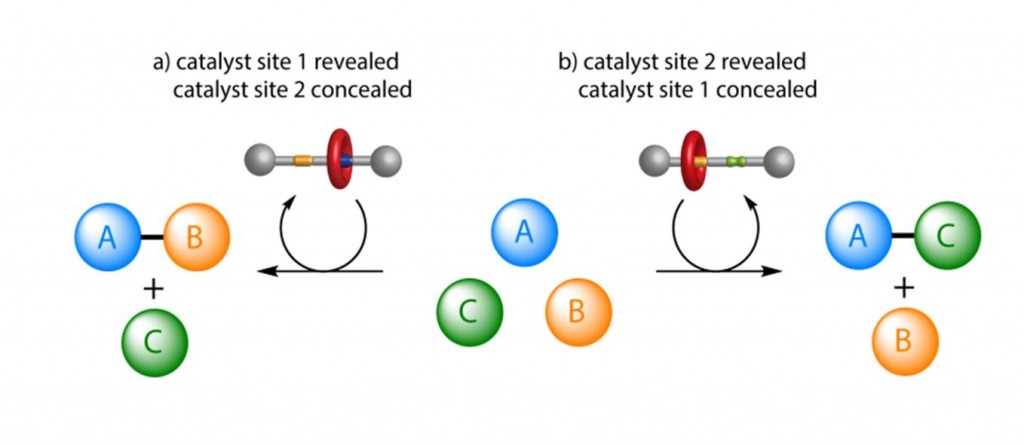
Switchable Rotaxane Organocatalyst – the position of the macrocycle either blocks or reveals one of the catalytic sites, leading to different products being formed from the same mixture of building blocks
The developed system promotes Michael addition reactions through iminium ion or hydrogen-bond-activated catalysis. The switch between these modes is provided by acid-base control of the position of the rotaxane macrocycle and leads to different products being formed.
This elegant catalytical switch approach holds great promise for chemical transformation and organic synthesis generally. To read the details of the transformations and, more importantly, how to make the rotaxane, read the Chemical Science paper today!
Read this Open Access Chem Sci article in full:
Selecting Reactions and Reactants using a Switchable Rotaxane Organocatalyst with Two Different Active Sites
David A Leigh, Jack Beswick, Victor Blanco, Guillaume De Bo, Urszula Lewandowska, Bartosz Lewandowski and Kenji Mishiro
Chem. Sci., 2014, Accepted Manuscript
DOI: 10.1039/C4SC03279A, Edge Article










