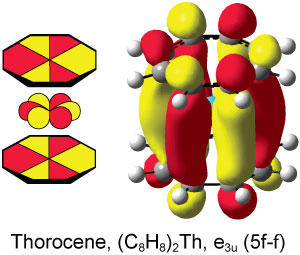
Theory had predicted the presence of Φ interactions in actinide systems but it had never been observed experimentally, until now. Scientists in the US using high-energy x-ray spectroscopy to study the involvement of the f-orbitals in actinide sandwich complexes have experimental evidence for this unusual interaction in thorocene.
At its most basic level, bonding in actinide molecules is typically comprised of a small amount of covalent orbital mixing in the presence of overwhelming ionic attractions. However, in many cases it is proposed that these small changes in f-element covalency are responsible for profound changes in chemical reactivity and actinide properties.
Covalency is a fundamental concept used to describe how elements share electrons in chemical bonds. For the d-block transition metal series, 3d, 4d, and 5d orbitals extend well into the periphery of the atom and can interact with valence orbitals of ligand atoms to form covalent chemical bonds. In contrast, the 4f orbitals of lanthanides are very core-like and their interactions with ligands are – in general – assumed to be of comparatively little chemical consequence. The actinide elements lie between these two extremes, and the extent to which valence f and d orbitals participate in chemical bonding is a subject of debate in the community.
You can also read this article in Chemistry World
Read the original journal article in Chemical Science:
New evidence for 5f covalency in actinocenes determined from carbon K-edge XAS and electronic structure theory
Stefan G. Minasian, Jason M. Keith, Enrique R. Batista, Kevin S. Boland, David L. Clark, Stosh A. Kozimor, Richard L. Martin, David K. Shuh and Tolek Tyliszczak
Chem. Sci., 2014, Advance Article
DOI: 10.1039/C3SC52030G, Edge Article