Potential energy surfaces of reactions are usually measured in the gas phase. However the vast majority of chemical reactions in the laboratory are conducted in solvent. What effect do these solvent molecules have on the potential energy surfaces that have been measured in the gas phase? Andrew Orr-Ewing and colleagues have been finding out.
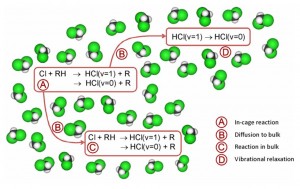
They have used ultrafast time-resolved broadband infra-red absorption spectroscopy to study the reaction of chlorine atoms with hydrocarbons in chlorinated solvent.
Two timescales were found, which corresponded to prompt reaction of the chlorine atom with the hydrocarbon, which was most likely located in the immediate solvent shell, and a slower reaction following diffusion into the bulk solvent. The presence of solvent molecules also partially suppresses the presence of vibrationally excited products that occur when the exothermic reaction is sufficient to form vibrationally hot products.
This work extends previous experiments conducted with cyanide radicals in solvent and general conclusions on the effect of solvent molecules on the potential energy surface are now emerging.
To find out more, download the Chemical Science article today (free to access until the 7th of December 2012).