Viable alternatives to fossil fuels are a vital area of research for chemists as current deposits dwindle. To combat our reliance on these fuels, US scientists have discovered a new route for turning the carbohydrate cellulose – the most abundant organic molecule on Earth – into 5-(hydroxymethyl)furfural (HMF), a promising precursor molecule to alternative fuels.
Whereas conventional methods for converting carbohydrates into HMF have involved the use of harsh reaction conditions and toxic heavy metal catalysts, the route proposed by Ronald Raines and co-workers at the University of Wisconsin-Madison uses a one-pot, low temperature approach that utilises less toxic organocatalysts instead.
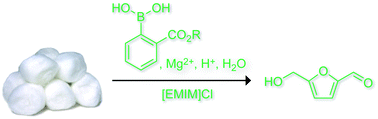
Converting cellulose to HMF is a three-step process. It consists of hydrolysis of cellulose to glucose, isomerisation of glucose to fructose and dehydration of fructose to HMF. The hardest step is the transformation from glucose to fructose and it is difficult to achieve this without using a catalyst. So, the team used a phenylboronic acid organocatalyst combined with magnesium chloride and mineral acids to get HMF in yields of up to 54%, a yield comparable to using toxic heavy metal catalysts. Phenylboronic acids have some catalytic activity, but the magnesium chloride and mineral acids are needed to boost the efficiency of the conversion process.
View the whole Chemistry World article…
Read the Chemical Science paper in full:
Organocatalytic conversion of cellulose into a platform chemical
Benjamin R. Caes , Michael J. Palte and Ronald T. Raines
Chem. Sci., 2013, DOI: 10.1039/C2SC21403B