You can have too much of a good thing, in a manner of speaking. Cisplatin, a platinum-based anticancer drug, has been hugely successful in treating a range of cancers but severe side-effects require its administration to be dose-limited. Analogues, such as carboplatin and nedaplatin have been developed to overcome these problems; carboplatin can be administered at 20 times the dosage of cisplatin. These drugs, however, have their own problems and exhibit inherent and acquired toxicity.
Investigation into the uptake of platinum anticancer drugs has highlighted that the regulation of transport using carriers/ channel-mediated systems may be one of the key factors for drug resistance. In particular, copper transporters and chaperones have been identified as participating in the uptake, transport and efflux of these drugs.
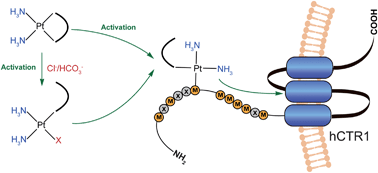
Hongzhe Sun and co-workers have advanced on previous work in which they employed NMR to study metallodrug-biomolecule interactions. They identified that the methionine residues in the N-terminus of human CTR1 (hCTR1_N), a copper transporter, were essential to allow binding to cisplatin, potentially activating the drug.
Looking at carboplatin and nedaplatin as well as cisplatin’s clinically ineffective isomer, transplatin, the research team extended their studies to further understand the interaction between platinum compounds with hCTR1_N and compare their findings with cisplatin.
The NMR studies revealed that carboplatin and nedaplatin both bound to the methionine residues of the transporter, although when compared to cisplatin, less was activated. Interestingly, they are more stable than cisplatin, potentially due to shielding effects of their ligands. Transplatin was also found to bind to methionine residues at a much higher rate than its isomer. This sophisticated investigation into the kinetics and speciation of platinum-based drugs gives greater understanding into drug-binding and insights into their distinct biological roles.
Read more about this research in Sun’s Chemical Science Edge article, free to access for a limited period.
Posted on behalf of Sarah Brown, Chemical Science web writer.