Researchers from the University of California have used computational studies to uncover the mechanism of a key step in their synthesis of a powerful toxin.
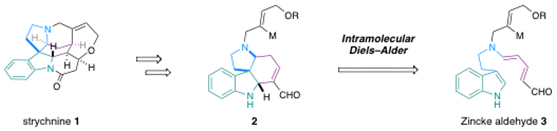
Last year, Christopher Vanderwal’s group published a very elegant and efficient approach to strychnine (1), resulting in the shortest synthesis of this alkaloid to date. An intramolecular Diels–Alder reaction of a Zinke aldehyde (3) provided tetracyclic intermediate (2) as a single diastereoisomer.
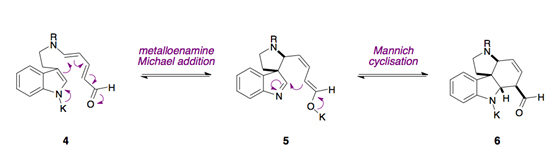
Computational modelling, performed in collaboration with K. Houk’s group, provided evidence that stepwise anionic cycloadditions were operational as opposed to a concerted Diels–Alder reaction.
The presence of a potassium base was found to be essential for the stepwise Michael/Mannich cascade to occur. The potassium cation is thought to organise the aldehyde into the appropriate conformation for the initial Michael reaction to occur. Additionally, the formation of a stable potassium enolate is thought to be the driving force for the Mannich reaction. Subtle changes in the reaction conditions therefore influence the preference for the formation of 6 over cycloreversion to form 4.
The researchers are hopeful that these interesting findings will enable the application of related Diels–Alder reactions to a wider range of substrates.