Computation experiments by researchers from the University of Geneva have illuminated their understanding of chemistry they previously reported and, importantly, have led to an improvement of the original methodology.
Alexandre Alexakis’ group developed a copper-catalysed asymmetric allylic alkylation, which transforms a racemic starting material into an enantioenriched product.
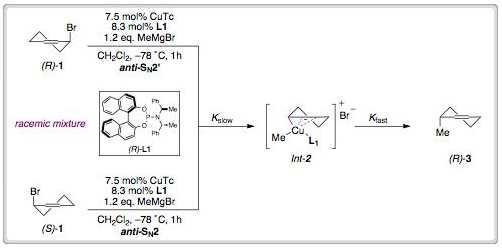
Computational modelling led to a revision of the original mechanistic explanation for the reaction outcome. The researchers propose that each enantiomer of the starting material undergoes divergent reactivity, where (R)-1 reacts through anti-SN2’ oxidative addition whilst its antipode (S)-1 reacts through anti-SN2. The regiodivergent oxidative addition leads to the formation of a common Cu(III) intermediate 2, which undergoes rapid reductive elimination to give the product (R)-3.
This work clearly demonstrates the importance of acertaining mechanistic insight in order to improve the practical application of organic chemistry.
Download Alexakis’ Edge article to find out more.