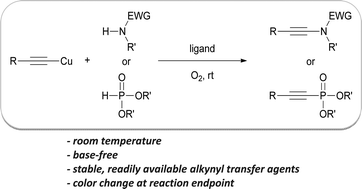
A mild and efficient procedure for the oxidative alkynylation of N- and P-based nucleophiles has been devised by scientists in France to make ynamides and alkynylphosphonates. These versatile alkynes add functionality to organic molecules and are used in medicinal chemistry and materials science. The heteroatom polarises the triple bond, allowing for highly regio- and stereoselective transformations. An advantage of the method is that it does not require a base, thermal activation, an inert atmosphere or expensive chemicals.
Reference:
Click-Alkynylation of N- and P- Nucleophiles by Oxidative Cross-Coupling with Alkynylcopper Reagents: A General Synthesis of Ynamides and Alkynylphosphonates.
K Jouvin, J Heimburger and G Evano, Chem. Sci., 2012
DOI: 10.1039/c2sc00842d