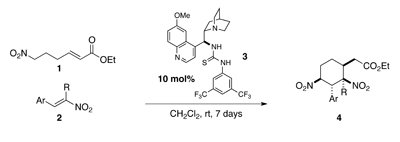
Researchers from the University of Reading have designed an organocatalysed cascade reaction for the construction of nitrocyclohexanes 4.
This elegant domino reaction enables the union of two achiral reagents to generate products containing up to five contiguous stereocentres in excellent levels of enantio- and diastereoselectivity.
André Cobb’s group employed a thiourea catalyst 3 to initiate a Michael-Michael cascade reaction between nitro-esters 1 and nitro-styrenes 2. The catalyst is thought to first coordinate to the nitro-ester prior to intramolecular deprotonation at the α-position to generate a nitronate. Synchronous coordination with nitrostyrene enables the first stereoselective Michael addition to generate a second nitronate primed for cyclisation onto the conjugated ester.
This cascade process demonstrates the power of organocatalysis for the asymmetric assembly of complex molecular architecture from simple starting materials.
Read more – download Cobb’s Edge article.
The asymmetric synthesis of densely functionalised molecules with up to five contiguous stereocentres in one pot has been a difficult task until now. Our simple new cascade reaction has achieved just this with the synthesis of complex nitrocyclohexanes in excellent stereocontrol. This methodology has then been successfully employed in the synthesis of an alpha-lycorane derivative which is of potential medicinal interest.
Sundaram Rajkumar, from the Cobb group