Some Alzheimer’s drugs work by blocking the activity of acetylcholinesterase, an enzyme that degrades the neurotransmitter acetylcholine to choline. To find new enzyme inhibitors, researchers need to identify choline formation, or the loss of acetylcholine, so they can tell whether the enzymatic reaction has stopped. But, acetylcholine and choline are both quaternary ammonium ions with very similar structures, making it difficult to distinguish between them.
To overcome this problem, teams led by Werner Nau at Jacobs University Bremen, Germany, and Yu Liu at Nankai University, China, have combined two sequential enzymatic reactions with a calixarene macrocycle that binds to a fluorescent dye to make a tandem assay that can screen for new inhibitors. The enzymes are highly specific and only work on one substrate.
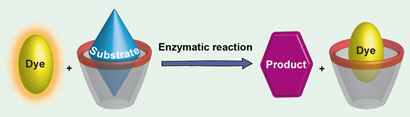
The tandem reaction involves a fluorescence ''switch-on'' displacement assay as a sensor for specific analytes
In their assay, they use acetylcholinesterase to turn acetylcholine to choline. A second enzyme – choline oxidase – turns the choline into betaine. While choline and betaine are similar, they have different affinities for binding within the calixarene. Because of this difference, the dye can replace the betaine inside the calixarene. This turns off the dye’s fluorescence, which is easy to detect. If the enzymatic reactions are inhibited, no betaine will be produced and so the dye’s fluorescence stays on……
To read the full story, please visit the Chemistry World website or download the Chemical Science Edge Article, which is free to access until the end of 2011!










