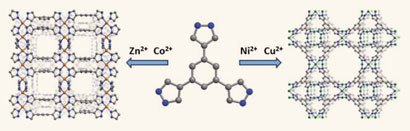
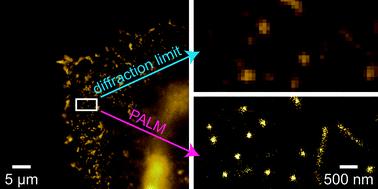
Cell membranes are made up of a mixture of different lipids and proteins. Membrane lipids aren’t randomly distributed but instead form raft-like microdomains. These are thought to provide platforms for protein interaction allowing protein trafficking and cell signalling.
Scientists have found it difficult to study these microdomains because their reported size is smaller than the resolution of light microscopy. Now a team of scientists from Japan and Belgium have circumvented this limitation by designing probes for cholesterol and sphingolipid-enriched microdomains for use in superresolution microscopy.
Read more:
Fluorescent probes for superresolution imaging of lipid domains on the plasma membrane
Hideaki Mizuno, Mitsuhiro Abe, Peter Dedecker, Asami Makino, Susana Rocha, Yoshiko Ohno-Iwashita, Johan Hofkens, Toshihide Kobayashi and Atsushi Miyawaki, Chem. Sci., 2011, DOI: 10.1039/C1SC00169H
Johan Hofkens, one of the authors of this manuscript, is a plenary speaker at the forthcoming Challenges in Chemical Biology (ISACS5) meeting. Register by 24th June to hear him speak.











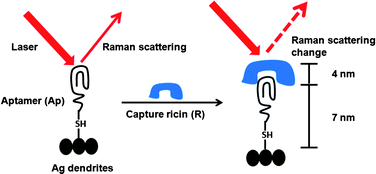 Ricin is a protein toxin naturally present in the castor bean plant (Ricinus communis); it is classified as a ‘select’ bioterror agent and was involved in a recent bioterrorism attack plot targeting US hotels and restaurants at multiple locations, as reported by the Department of Homeland Security officials in December 2010.
Ricin is a protein toxin naturally present in the castor bean plant (Ricinus communis); it is classified as a ‘select’ bioterror agent and was involved in a recent bioterrorism attack plot targeting US hotels and restaurants at multiple locations, as reported by the Department of Homeland Security officials in December 2010.


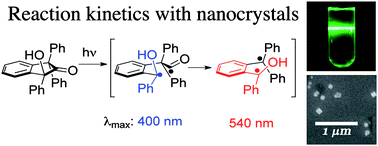
 On Monday I attended the
On Monday I attended the