We are very pleased to introduce Professor Takashi Morii, who is the corresponding author of the RSC Advances article, A two-step screening to optimize the signal response of an auto-fluorescent protein-based biosensor. The manuscript was well received by reviewers and was handpicked by our reviewers and handling editors to be part of our Popular Advances collection.
Professor Mori told us more about the work that went into this article and what he hopes to achieve in the future. You can explore other articles in our 2022 Popular Advances online collection here.
Meet the author:
Takashi Morii was born in 1959 in Hyogo, Japan. He studied Chemistry at Kyoto University (B. Eng., 1982, Ph.D. 1988) with Prof. T. Matsuura and Prof. I. Saito. He conducted postdoctoral research with Prof. J. K. Barton at Columbia University and California Institute of Technology. In 1992, he was appointed as an Assistant Professor at Kyoto Institute of Technology and subsequently moved to Institute for Chemical Research at Kyoto University. In 1998, he moved to Institute of Advanced Energy, Kyoto University, where he was promoted to Professor in 2005.
Could you briefly explain the focus of your article to the non-specialist (in one or two sentences only) and why it is of current interest?
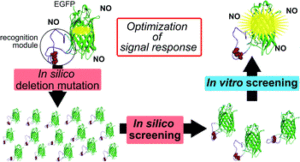
Construction of an auto-fluorescent protein (AFP)-based biosensor consisting of a recognition, or a reaction, module and AFP often encounters difficulty owing to the lack of structural information for the recognition module and requirement of laborious tasks for functional optimization. This study describes a two-step screening strategy that allows facile optimization of the optical response of AFP-based biosensor for nitric oxide (NO), which is also applicable for many types of AFP-based biosensors.
How big an impact could your results potentially have?
Our two-step, first in silico and second in vitro, screening strategy provides a convenient and high-throughput screening method for the optimization of the signal response of AFP-based biosensors. Especially, our strategy has an advantage for cases when the detailed information on the structural change of recognition module is not available. AFP-based biosensors are quite useful in visualizing the dynamics of cellular important factors because of their suitability for high spatiotemporal resolution and long-time imaging. Our strategy would accelerate the development of various types of biosensors for the factors of interest in the cell.
Could you explain the motivation behind this study?
We have previously constructed a fusion of a segment of the putative NO-sensing module of the TRPC5 channel with enhanced green fluorescent protein (EGFP) to evaluate this putative NO-induced structural change in TRPC5. While the construct successfully detected the putative structural change by the reaction with NO as a change in the fluorescence intensity ratio of EGFP, the observed response was quite weak. We considered that the TRPC5 loop-EGFP construct could be converted to a cellular NO sensor by enhancing its response through the mutation and screening. In addition, developing a general strategy to construct AFP-based biosensors that visualize various kinds of second messengers would promote further investigation of signal transduction.
In your opinion, what are the key design considerations for your study?
An AFP-based biosensor is designed by conjugating an appropriate recognition or reaction module for a given target to an AFP transduction module. Structural changes in the recognition module induced by the recognition/reaction event are transduced to a change of fluorescence signal of AFP. To obtain usable AFP-based biosensors, many sensor candidates must be constructed and evaluated their responses, which are time consuming and required laborious tasks. We consider that a screening to select candidates showing larger structural changes at the reaction module upon the reaction based on in silico simulation in the first step would reduce these tasks. Structural change of the reaction modules of candidates are evaluated by root-mean-square-deviation (RMSD) of the coordinates for the backbone of reaction module between before and after the reaction based on in silico simulation.
Which part of the work towards this paper proved to be most challenging?
The most challenging part of this work is whether the in silico screening evaluated by using the RMSD values could select candidates with reasonable signal responses because it is very difficult to predict the exact structural change of candidates upon the reaction in silico. Fortunately, the second in vitro screening revealed that RMSD values could successfully provide indexes for the signal response of the candidates, although large RMSD values did not always correspond to the large signal response.
What aspect of your work are you most excited about at the moment?
It was quite exciting to find that the sensor candidates from the first in silico screening showed enhanced signals in the in vitro second screening. It was also exciting to confirm that a construct obtained from the two-step screening showed a reasonable signal response in living mammalian cells. This result demonstrated that our screening strategy can be applied to enhance the signal response sufficient for cellular applications.
What is the next step? What work is planned?
The reaction module of selected AFP-based biosensor changes its structure upon formation of a disulphide bond to emit the signal. We anticipated a certain selectivity for the disulphide bond formation by NO, but apparently the selected AFP-based biosensor showed similar response to NO and H2O2. The next step is to develop a convenient strategy to install a selectivity to NO and H2O2 on the AFP-based biosensor selected in this work.
A two-step screening to optimize the signal response of an auto-fluorescent protein-based biosensor
Shunsuke Tajima,a Eiji Nakata,a Reiko Sakaguchi,b Masayuki Saimura,a Yasuo Moric and Takashi Morii*a
RSC Adv., 2022,12, 15407-15419
Submit to RSC Advances today! Check out our author guidelines for information on our article types or find out more about the advantages of publishing in a Royal Society of Chemistry journal.
Keep up to date with our latest Popular Advances, Reviews, Collections & more by following us on Twitter. You can also keep informed by signing up to our E-Alerts.