We are very pleased to introduce Jamie Antonio Portilla Salinas, corresponding author of the paper ‘Pyrazolo[1,5-a]pyrimidines-based fluorophores: a comprehensive theoretical-experimental study‘. His article has been very well received and handpicked by our reviewers and handling editors as one of our October HOT articles. Jamie told us more about the work that went into this article and what he hopes to achieve in the future. You can find out more about the author and his article below and find more HOT articles in our online collection.
Meet the authors
Jaime Portilla studied for a degree and PhD at The Universidad del Valle located in Cali-Colombia, his birthplace. He carried out a PhD supervised by Jairo Quiroga on synthesis of 5-aminopyrazoles and their reaction with 1,3-bis-electrophilic compounds under eco-compatible strategies. After the award of his PhD in November 2007, he moved to Bogotá (January 2008) and was appointed as a lecturer in organic chemistry at The Universidad de los Andes. He was promoted to Associate Lecturer in 2011 and since August 2018 Jaime Portilla is ‘Associate Professor III’.
The Prof. Jaime Portilla group’s research (Bioorganic Compounds Research Group) focuses on eco-compatible organic synthesis strategies, with a particular interest in aza-heterocyclic compounds synthesis of biological and photophysical potential. (Source: ORCID.)
Could you briefly explain the focus of your article to the non-specialist (in one or two sentences only) and why it is of current interest?
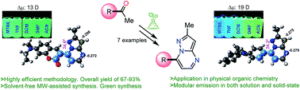
Here we developed a new family of fluorescent molecules with interesting features such as easy to synthesize (and easily functionalizable) with excellent green chemistry performance, starting from low-cost raw materials and with outstanding photophysical properties in both solution and solid-state.
How big an impact could your results potentially have?
These results could be the beginning of the inclusion of sustainable parameters in the design of fluorescent probes for materials, chemosensors and/or biological applications.
Could you explain the motivation behind this study?
Recently, fluorescent compounds with biological activity have received attention due to the possibility of monitoring those compounds in biological media such as cells and biofluids. Our research group is working on designing new pharmacophores for cancer treatment based on pyrazolo[1,5-a]pyrimidines and we do believe that the incorporation of remarkable photophysical properties will be important for the study of key parameters such as the distribution of the biologically active compound inside the body and even at subcellular levels.
In your opinion, what are the key design considerations for your study?
From the optical properties for pyrazolo[1,5-a]pyrimidines described in our previous works, we decided to incorporate green chemistry principles due to needing sustainable research according to the United Nations Agenda 2030. Thus, these fluorophores well-known for their synthetic versatility and important biological properties were designed and obtained via a eco-friendly approach. In addition, the effect of a modulable donor groups at position 7 on the heterocyclic core was corroborated by computational calculations, which would contribute towards an intelligent design of ‘highly functional fluorophores’.
Which part of the work towards this paper proved to be most challenging?
The work most challenging was to find a good correlation of the experimental results with the theoretical calculations, since the optical phenomena are strongly governed by the microenvironment of the analyzed molecule.
What aspect of your work are you most excited about at the moment?
Here we identify compounds with excellent optical properties (QYSS up to 63%) emitting in the blue region, a color highly interesting in OLED’s research field. These results are remarkable and further research is ongoing in this direction.
What is the next step? What work is planned?
The results show us the possibility to functionalized the ring at position 4 (nitrogen atom) to generated pyrazolo[1,5-a]pyrimidine salts. At this moment we had found outstanding results in the field of anion detection using this approach. Once position 7 showed excellent results, we would like to explore the other positions on the core such as 2, 3 and 5. Also, the introduction of highly polar groups will improve the water solubility and exciting applications are expected from it. Some of these probes and related compounds will be tested in antitumor assays. And of course, to continue the study of some derivatives published here specifically in their device performance construction (OLED).
Pyrazolo[1,5-a]pyrimidines-based fluorophores: a comprehensive theoretical-experimental study
Alexis Tigreros, Sandra-L. Aranzazu, Nestor-F. Bravo, Jhon Zapata-Rivera and Jaime Portilla
RSC Adv., 2020,10, 39542-39552
DOI: 10.1039/D0RA07716J, Paper
 Submit to RSC Advances today! Check out our author guidelines for information on our article types or find out more about the advantages of publishing in a Royal Society of Chemistry journal.
Submit to RSC Advances today! Check out our author guidelines for information on our article types or find out more about the advantages of publishing in a Royal Society of Chemistry journal.
Keep up to date with our latest HOT articles, Reviews, Collections & more by following us on Twitter. You can also keep informed by signing up to our E-Alerts.