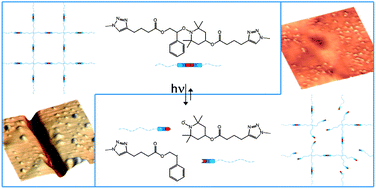
In this study, Poly, Lalevée, Matyjaszewski and co-workers reported on a new approach for the synthesis of dynamic covalent networks exhibiting self-healing properties under UV irradiation. The procedure combined ATRP and click chemistry as two versatile synthetic tools for the design of well-defined peripherally functionalized star-like oligomers followed by their covalent bonding with responsive alkoxyamine crosslinking agents. The incorporation of alkoxyamine linkages into the junctions between the stars enabled their subsequent cleavage under irradiation. Beyond self-healing materials, the concept developed in the present study might be of interest regarding aging issues of polymeric materials due to UV radiation.
Introduction of self-healing properties into covalent polymer networks via the photodissociation of alkoxyamine junctions by Siham Telitel, Yoshifumi Amamoto, Julien Poly, Fabrice Morlet-Savary, Olivier Soppera, Jacques Lalevée and Krzysztof Matyjaszewski Polym. Chem. 2014, 5, 921-930.
Julien Nicolas is a web-writer and advisory board member for Polymer Chemistry. He currently works at Univ. Paris-Sud (FR) as a CNRS researcher.