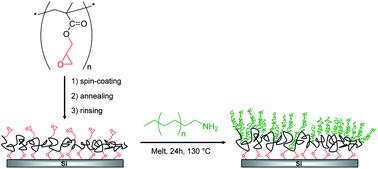
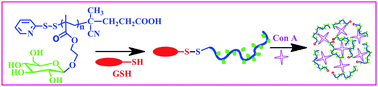
A poly(glycidyl methacrylate) (PGMA) synthesized by RAFT polymerization was spin-coated onto a silicon substrate to yield, after annealing and rinsing unreacted chains, covalently attached epoxy-containing PGMA pseudo-brushes. A tailor-made ω-amino-poly(ethylene) (Mn = 1040 g mol−1, PDI = 1.3) was then grafted in melt at 130 °C for 24 h by reaction between amine chain-ends and surface-tethered epoxy groups. After discarding unreacted poly(ethylene) chains by sonication and Soxhlet rinsing, the resulting poly(ethylene) brushes were characterized by scanning probe microscopy, water contact angle and neutron reflectivity measurements. The grafting of poly(ethylene) brushes to silicon substrates has thus been demonstrated for the first time and resulted in nanostructured grafted layers with homogeneous surface coverage.
Poly(ethylene) brushes grafted to silicon substrates by Denis Damiron, Jérôme Mazzolini, Fabrice Cousin, Christophe Boisson, Franck D’Agosto and Eric Drockenmuller Polym. Chem., 2012, 3, 1838-1845.
To keep up-to-date with all the latest research, sign up for the journal’s e-alerts or RSS feeds or follow Polymer Chemistryon Twitter or Facebook.