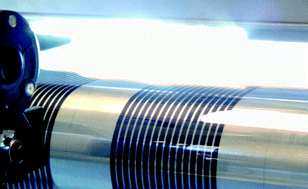
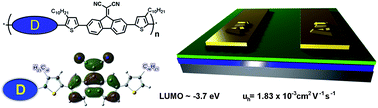
Light induced thermocleaving of a thermally reactive copolymer based on dithienylthiazolo[5,4-d]thiazole (DTZ) and silolodithiophene (SDT) in contact with the heat sensitive substrate polyethyleneterphthalate (PET) was effectively demonstrated with the use of high intensity pulsed light, delivered by a commercial photonic sintering system. Thermally labile ester groups are positioned on the DTZ unit of the copolymer that can be eliminated thermally for enhanced photochemical stability and advantages in terms of processing (solubility/insolubility switching). The photonic sintering system was successfully implemented in a full roll-to-roll process on flexible PET substrates and large-area polymer solar cell modules were prepared by solution processing of five layers under ambient conditions using the photonic sintering system for thermocleaving of the active layer. The PET foil did not show any deformation after exposure to the high intensity light only leaving the insoluble thermocleaved active layer. The active layer remained planar after light exposure thereby allowing the coating of supplementary material on top.
Rapid flash annealing of thermally reactive copolymers in a roll-to-roll process for polymer solar cells, Martin Helgesen , Jon Eggert Carlé , Birgitta Andreasen , Markus Hösel , Kion Norrman , Roar Søndergaard and Frederik C. Krebs Polym. Chem., 2012,3, 2649-2655.
To keep up-to-date with all the latest research, sign up for the journal’s e-alerts or RSS feeds or follow Polymer Chemistryon Twitter or Facebook.