Lehnen et al. highlight the role of reversible deactivation as a key difference between photo-iniferter and conventional RAFT polymerization.
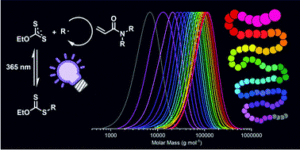
The use of light has become increasingly widespread in diverse polymerization approaches including reversible-addition fragmentation chain-transfer (RAFT) strategies. Among these, the photo-iniferter (PI)-RAFT polymerization in which light directly activates the chain transfer agent (CTA), has been shown to overcome several of the restrictions of conventional RAFT resulting in increased chain end fidelity. In this context, reversible deactivation is accepted to determine the fate of the growing radical via pathways that need to be understood to offer the means to further push the limits of PI-RAFT polymerization.
To address this, Hartlieb and collaborators studied the PI-RAFT using an acrylamide (N-acryloyl morpholine) and a xanthate ((2-((ethoxycarbonothioyl)thio)propionic acid)). This monomer-CTA pair combination was selected on the basis of the low chain transfer capabilities (Ctr < 1) expected to result in high dispersities (>1.5). When targeting different degrees of polymerization (DP), the control over the molecular weight distribution was not found to significantly increase. However, control could be achieved through slow monomer addition that results in increasing the numbers of activation-deactivation events per monomer addition. Importantly, the high livingness associated with PI-RAFT proved to be invaluable in chain extension experiments since it was found to enable the straightforward, easy and rapid synthesis of very high molecular weight multiblock copolymers with up to 20 blocks and a high number of repeating units per block (DP = 25-100) with impressive precision.
In summary this study highlights the role of reversible deactivation and employs the high livingness of PI-RAFT to demonstrate its enormous potential for the synthesis of polymeric materials and more specifically segmented macromolecules.
Tips/comments directly from the authors:
- We want to emphasize how fast and easy polymerization reactions can be performed using this technique as the shown xanthate is an extremely powerful iniferter
- The shown multiblocks were produced in a very straight forward way; no rigorously clean or inert conditions or specialized equipment.
- The photo-iniferter process is older than RAFT polymerization but its full potential isn’t used yet.
The difference between photo-iniferter and conventional RAFT polymerization: high livingness enables the straightforward synthesis of multiblock copolymers, Polym. Chem., 2022, 13, 1537-1546
Link to the paper: https://pubs.rsc.org/en/content/articlelanding/2022/py/d1py01530c
Link to Dr Matthias Hartlieb’s group website: https://www.uni-potsdam.de/polybio
You can follow Dr Matthias Hartlieb on Twitter: @PolyBioPotsdam.
 Dr. Kelly Velonia is an Advisory Board Member and a Web Writer for Polymer Chemistry. She joined the Department of Materials Science and Technology in 2007. Research in her group focuses on the synthesis and applications of bioconjugates and biopolymers.
Dr. Kelly Velonia is an Advisory Board Member and a Web Writer for Polymer Chemistry. She joined the Department of Materials Science and Technology in 2007. Research in her group focuses on the synthesis and applications of bioconjugates and biopolymers.