Here are the most-read Polymer Chemistry articles for April 2011:
Construction of mixed micelle with cross-linked core and dual responsive shells
Cong Chang, Hua Wei, Qian Li, Bin Yang, Ni Chen, Jin-Ping Zhou, Xian-Zheng Zhang and Ren-Xi Zhuo, Polym. Chem., 2011, 2, 923-930
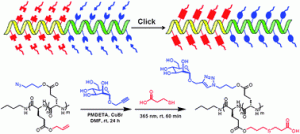
Synthesis of thermoresponsive oxazolone end-functional polymers for reactions with amines using thiol-Michael addition “click” chemistry
The Hien Ho, Martin Levere, Jean-Claude Soutif, Véronique Montembault, Sagrario Pascual and Laurent Fontaine, Polym. Chem., 2011, 2, 1258-1260
Overcoming the PEG-addiction: well-defined alternatives to PEG, from structure–property relationships to better defined therapeutics
Matthias Barz, Robert Luxenhofer, Rudolf Zentel and María J. Vicent, Polym. Chem., 2011, Advance Article, DOI: 10.1039/C0PY00406E
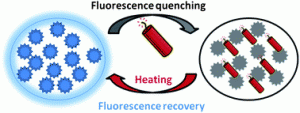
Photo-responsive, biocompatible polymeric micelles self-assembled from hyperbranched polyphosphate-based polymers
Chaojian Chen, Gongyan Liu, Xiangsheng Liu, Shaopeng Pang, Congshan Zhu, Liping Lv and Jian Ji, Polym. Chem., 2011, 2, 1389-1397
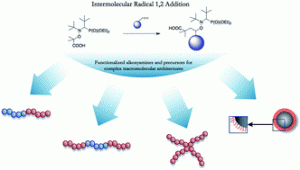
Polymeric nanomaterials from combined click chemistry and controlled radical polymerization
Rong Fu and Guo-Dong Fu, Polym. Chem., 2011, 2, 465-475
Optimizing the generation of narrow polydispersity ‘arm-first’ star polymers made using RAFT polymerization
Julien Ferreira, Jay Syrett, Michael Whittaker, David Haddleton, Thomas P. Davis and Cyrille Boyer, Polym. Chem., 2011, Advance Article, DOI: 10.1039/C1PY00102G
Thiol-ene “click” reactions and recent applications in polymer and materials synthesis
Andrew B. Lowe, Polym. Chem., 2010, 1, 17-36
Surface modification of carbon nanotubes with dendrimers or hyperbranched polymers
Jiao-Tong Sun, Chun-Yan Hong and Cai-Yuan Pan, Polym. Chem., 2011, 2, 998-1007
Functionalization of inorganic nanoparticles with polymers for stealth biomedical applications
Koon Gee Neoh and En Tang Kang, Polym. Chem., 2011, 2, 747-759
New micellar morphologies from amphiphilic block copolymers: disks, toroids and bicontinuous micelles
Simon J. Holder and Nico A. J. M. Sommerdijk, Polym. Chem., 2011, 2, 1018-1028
To keep up-to-date with all the best Polymer Chemistry research articles, sign up for the journal’s e-alerts here.