pH-sensitive anionic dyes have been widely used in scientific research and industrial applications. The most common pH test approach generally utilizes papers as the substrate which absorbed pH-sensitive dyes. However, pH test papers often suffer from the leaching of dyes into solution, and the contamination of the samples which can result in anomalous readings. In the past years, plastic pH-sensitive strips have been prepared by immobilizing small molecular pH-sensitive dyes into the polymer matrix via the adsorption or entrapment approach. However, the physical entrapment may lead to a gradual loss of dyes from the substrates, which therefore limits the sensor stability and long-term practical applications. Therefore, various polymers with covalently bonded pH-sensitive moieties have been recently developed for the pH test.
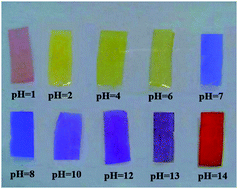
In their study, Yan and co-workers reported a facile and effective strategy for the preparation of plastic pH indicator strips and the characterization of their pH sensitivity. They focused their attention on poly(ionic liquids) (PILs) due to their excellent ion exchange capability, enabling the preparation of PILs with a variety of counteranions by polymerization of only one IL monomer and followed by anion-exchange reactions. The pH-sensitive strips were prepared via the cross-linking of imidazolium type IL monomers with acrylonitrile and followed by anion-exchange with sulfonated anionic dyes which bear their negative charge in a wide pH range. The resultant pH indicator strips exhibited enhanced pH-responsive colour changes and robust pH-response reversibility in both aqueous and organic solutions.
Plastic reusable pH indicator strips: preparation via anion-exchange of poly(ionic liquids) with anionic dyes by Jiangna Guo, Lihua Qiu, Zhijun Deng and Feng Yan, Polym. Chem., 2013, 4, 1309-1312.
Julien Nicolas is a guest web-writer for Polymer Chemistry. He currently works at Univ. Paris-Sud (FR) as a CNRS researcher.