April M. Kloxin, Ph.D., is an Associate Professor in Chemical & Biomolecular Engineering and Materials Science & Engineering at the University of Delaware (UD) and a member of the Breast Cancer Research Program at the Helen F. Graham Cancer Center and Research Institute in the Christiana Care Health System. She obtained her B.S. and M.S. in Chemical Engineering from North Carolina State University and Ph.D. in Chemical Engineering from the University of Colorado, Boulder, as a NASA Graduate Student Research Program Fellow. She trained as a Howard Hughes Medical Institute postdoctoral research associate at the University of Colorado before joining the faculty at UD in 2011. Her group aims to create unique materials with multiscale property control for addressing outstanding problems in human health. Her research currently focuses on the design of responsive and hierarchically structured soft materials and development of controlled, dynamic models of disease and regeneration. Her honors include the Biomaterials Science Lectureship 2019, ACS PMSE Arthur K. Doolittle Award 2018, a Susan G. Komen Foundation Career Catalyst Research award, a NSF CAREER award, and a Pew Scholars in Biomedical Sciences award.
What was your inspiration in working with polymers?
I have always enjoyed building things and had a desire to use those skills to help people. I discovered my passion for using chemical approaches to build soft polymeric materials possessing unique and useful properties as an undergraduate and Master’s student at North Carolina State University (NCSU). At NCSU, I had the opportunity to work in a collaborative environment with many extraordinary friends and colleagues having great polymer science and engineering expertise, including my MS thesis advisors Profs. Rich Spontak and Stuart Cooper. This experience helped me understand the connection between molecular design and synthetic approaches for building polymeric materials with specific properties for a desired application. I had the opportunity to fully realize and direct this passion working at the interface between polymeric materials and biological systems under the outstanding advisement and mentorship of Prof. Kristi Anseth at the University of Colorado, Boulder, for my Ph.D. and with the many remarkable researchers in her group and at the University.
What was the motivation behind your most recent Polymer Chemistry article?
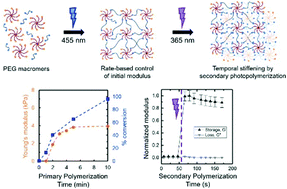
From a biological perspective, my group has a focus on understanding how changes in the structure, mechanical properties, and compositions of tissues in the human body that occur upon injury influence the function and fate of key cells in healing and disease. In this context, we have been interested in building synthetic mimics of these complex systems and processes, and we wanted to establish simple yet effective approaches for controlling the density and stiffness of soft materials when and where desired for hypothesis testing. In the Polymer Chemistry manuscript, we were inspired by the work of Prof. Matt Becker (Duke University) amongst others demonstrating how the rate of formation of water-swollen polymer networks, hydrogels, could be used to control defect formation, network heterogeneity, and thereby the mechanical properties of the resulting materials. We hypothesized that the rate-based control of properties that others observed with catalyzed step growth reactions was translatable to a photo-polymerized system, affording the implementation of a variety of photochemical controls (e.g., wavelength, intensity, time). In particular, by selecting a wavelength of light that was not centered at the maximum absorption of the photoinitiator, we were better able to control the rate of photopolymerization with an accessible bench-top visible light LED system and thereby defect formation. We then saw an opportunity to exploit dangling-end defects that were generated with this rate-based approach to increase crosslink density and ‘stiffen’ these materials with a secondary photopolymerization. We are excited about the potential that this light-triggered rate-based approach for controlling mechanical properties of polymer networks has for a number of applications, including our on-going studies of cell response to matrix stiffening.
Which polymer or materials scientists are you most inspired by?
Oh, there are so many! I am especially inspired by the work and leadership of Prof. Paula Hammond (MIT) and Prof. Kristi Anseth, who continue to blaze trials at the interface between polymers, materials, and biology to solve complex problems, and Prof. Chris Bowman (University of Colorado, Boulder) and the late Prof. Charlie Hoyle (University of Southern Mississippi), who have pioneered the use of light-triggered step growth reactions for creating polymeric materials with diverse and robust properties.
Can you name some up and coming polymer chemists who you think will have a big impact on the field?
It is an exciting time in polymer chemistry with many excellent researchers working from different perspectives to advance not only the field of polymer chemistry, but also to make fundamental breakthroughs that have an impact in biology, medicine, and energy. Selecting just a few is difficult in this context. A few that come to mind at the moment whose work I find particularly inspiring are Prof. Aaron Esser Kahn (University of Chicago) in biomolecular design of polymeric materials for rewiring the immune system, Prof. Dominik Konkolewicz (Miami University Ohio) in bioconjugations and dynamic covalent chemistries with polymeric materials, Prof. Rachel A. Letteri (University of Virginia) in peptide-polymer conjugates for multi-scale and dynamic properties, and my own new colleague Prof. Laure Kayser (University of Delaware) in conducting and semiconducting polymers.
How do you spend your spare time?
I enjoy making things, from designing materials at work to preparing satisfying meals in the kitchen at home. Breakfast foods are my favorite, and I have different recipes that I continue to hone on weekends for quick meals during the week. I also love being outside walking, hiking, or running with my friends or my husband and our two sons, particularly in the beautiful early autumn weather we currently are having.
What profession would you choose if you weren’t a chemist?
My obsession with the complexity of biological systems and improving human health would keep me in science and engineering, whether in molecular biology or bioinformatics or more applied in medicine.
Read April’s recent Polymer Chemistry article now for FREE until 31st October!
Rate-based approach for controlling the mechanical properties of ‘thiol–ene’ hydrogels formed with visible light
The mechanical properties of synthetic hydrogels traditionally have been controlled with the concentration, molecular weight, or stoichiometry of the macromolecular building blocks used for hydrogel formation. Recently, the rate of formation has been recognized as an important and effective handle for controlling the mechanical properties of these water-swollen polymer networks, owing to differences in network heterogeneity (e.g., defects) that arise based on the rate of gelation. Building upon this, in this work, we investigate a rate-based approach for controlling mechanical properties of hydrogels both initially and temporally with light. Specifically, synthetic hydrogels are formed with visible light-initiated thiol–ene ‘click’ chemistry (PEG-8-norbornene, dithiol linker, LAP photoinitiator with LED lamp centered at 455 nm), using irradiation conditions to control the rate of formation and the mechanical properties of the resulting hydrogels. Further, defects within these hydrogels were subsequently exploited for temporal modulation of mechanical properties with a secondary cure using low doses of long wavelength UV light (365 nm). The elasticity of the hydrogel, as measured with Young’s and shear moduli, was observed to increase with increasing light intensity and concentration of photoinitiator used for hydrogel formation. In situ measurements of end group conversion during hydrogel formation with magic angle spinning (MAS 1H NMR) correlated with these mechanical properties measurements, suggesting that both dangling end groups and looping contribute to the observed mechanical properties. Dangling end groups provide reactive handles for temporal stiffening of hydrogels with a secondary UV-initiated thiol–ene polymerization, where an increase in Young’s modulus by a factor of ∼2.5× was observed. These studies demonstrate how the rate of photopolymerization can be tuned with irradiation wavelength, intensity, and time to control the properties of synthetic hydrogels, which may prove useful in a variety of applications from coatings to biomaterials for controlled cell culture and regenerative medicine.
About the Webwriter
 Simon Harrisson is a Chargé de Recherche at the Centre National de la Recherche Scientifique (CNRS), based in the Laboratoire des IMRCP in Toulouse. His research seeks to apply a fundamental understanding of polymerization kinetics and mechanisms to the development of new materials. He is an Advisory Board member for Polymer Chemistry. Follow him on Twitter @polyharrisson
Simon Harrisson is a Chargé de Recherche at the Centre National de la Recherche Scientifique (CNRS), based in the Laboratoire des IMRCP in Toulouse. His research seeks to apply a fundamental understanding of polymerization kinetics and mechanisms to the development of new materials. He is an Advisory Board member for Polymer Chemistry. Follow him on Twitter @polyharrisson