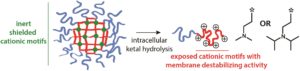
Engineering mannosylated nanomaterials with various functionalities can significantly contribute to the development of more effective vaccines or cancer immunotherapeutics that target immune cell subsets that express the mannose receptor. With this in mind, De Geest’s group aimed at equipping mannosylated nanogels with membrane-destabilizing properties that are responsive to the acidic pH found in intracellular vesicles, such as endosomes, but are shielded when the nanogels are intact in neutral pH. In particular, membrane destabilizing tertiary amine moieties were successfully introduced in the core of the nanogels. Subsequently and via using a pH-sensitive ketal-based crosslinker, the membrane-destabilizing properties only become activated upon pH-triggered disassembly of the nanogels into soluble unimers. In order to achieve this, the effect of tertiary amine modification of mannosylated block copolymers with N,N-dimethylamine (DMAEA) and N,N-diisopropylamine (DiPAEA) was initially evaluated. Both block copolymers showed strong haemolytic activity and the DiPAE block copolymers demonstrated an activity only at acidic endosomal pH values. To silence the membrane destabilizing activity and render the nanogels non-cytotoxic at high concentration, cross-linking of the block copolymers into nanogels was conducted. Interestingly, when a pH degradable ketal cross-linker was used, the nanogels could regain their activity by exposing them to mild acidic pH. As the authors nicely conclude, such synthetic mannosylated materials may hold promise for cytoplasmic delivery of non-membrane permeable therapeutic macromolecules.
Tips/comments directly from the authors:
- Dendritic cells and macrophages reside in peripheral tissue, lymphoid organs and sites of inflammation and tumor tissue. They are a primary therapeutic target.
- The use of tetraacetylated carbohydrate monomers allows for straightforward polymerization and work-up in organic media. Deacetylation is easily performed in a final step and yields hydrophilic glyconanogels.
- The use of a pentafluorophenyl activated ester hydrophobic polymer bock allows for self-assembly in aprotic polar solvents. This is ideal for successive post-modification steps without facing hydrolysis as a side reaction.
- Diisopropylamine motifs are highly efficient in destabilizing lipid membranes at acidic pH, presumably through hydrophobic interaction with phospholipid membranes.
Read this article for FREE until the 15th October!
Engineering mannosylated nanogels with membrane-disrupting properties Polym. Chem., 2019, 10, 4297-4307, DOI: 10.1039/C9PY00492K
About the Web Writer
Dr. Athin a Anastasaki is an Editorial Board Member and a Web Writer for Polymer Chemistry. Since January 2019, she joined the Materials Department of ETH Zurich as an Assistant Professor to establish her independent research group.
a Anastasaki is an Editorial Board Member and a Web Writer for Polymer Chemistry. Since January 2019, she joined the Materials Department of ETH Zurich as an Assistant Professor to establish her independent research group.