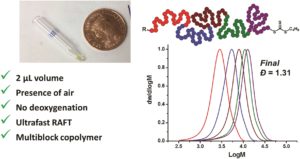
Oxygen is considered detrimental for radical polymerizations and as such traditional deoxygenation strategies (e.g. freeze pump thaw, nitrogen sparging, etc.) are typically required for the complete removal of oxygen. However, such methods may also possess drawbacks (e.g. lack of reproducibility) and as such alternative polymerization strategies that do not require external deoxygenation have been developed. To this end, Wilson, Perrier, Tanaka and co-workers reported the ultrafast polymerization of a range of acrylamide monomers in water exploiting reversible addition-fragmentation chain-transfer (RAFT) polymerization in the presence of air. The authors used microvolume insert vials as the reaction vessels and found that good control over the molecular weight and the dispersity could be maintained at very low volumes (down to 2 μl scale). Importantly, the resulting materials were successfully chain extended multiple times by sequential monomer additions allowing the facile synthesis of pentablock copolymers with a final volume of the reaction mixture not exceeding 10 μl. Nuclear magnetic resonance and gel permeation chromatography have been used to characterize the materials which were found to reach very high monomer conversions accompanied with low molecular weight distributions. These results demonstrate that RAFT polymerization can be used as a high-throughput screening method for the preparation of complex sequence-controlled multiblock copolymers. The authors are currently looking at expanding the scope of their investigation to include the synthesis of more complex structures and investigate their applicability to biological sciences.
Tips/comments directly from the authors:
- In general, the aqueous ultrafast RAFT conditions (Polym. Chem., 2015, 6, 1502-1511) used in our work can also be scaled up (> 50 ml), however, depending on the set up, it may take longer time to permit sufficient heat transfer.
- The protocol is limited to acrylamidic monomer family in solvent mixture that constitutes mostly water to permit ultrafast polymerisation open to air without prior deoxygenation with quantitative monomer conversion. In addition, changing RAFT agent with a more stabilizing R group requires some modification to the protocol due to a longer induction period.
- Scaling down works very well in microvolume inserts, using centrifuge to spin down the reaction mixture to the bottom. Caution has to be taken when spinning inserts/vials inside a centrifuge, as leaving it spinning for too long may break the vials.
- For sequential chain extensions, the reactions vessels were cooled with liquid nitrogen, which was admittedly an overkill. Instead, it can also be cooled with ice-water bath. Cooling between blocks is essential at microscale for premixing the sequential monomer solution and subsequent centrifuge is advised to spin down the mixture again before reheating.
- For multiple reactions, a piece of cardboard was punctured and used as a platform for multiple inserts to be conveniently placed in an oil bath at the same time.
- The master mix containing PATBC (the RAFT agent) to target DP25, may appear somewhat cloudy with only 20% dioxane (of the total solvent volume added) especially when cooled or stored in refrigerator, however it will turn clear upon heating.
- When targeting high DP (>100), although some dioxane was used in our paper, the monomer (DMA, NAM) can sufficiently solubilise the PABTC without any co-organic solvents.
Read the full Open Access article: Microscale synthesis of multiblock copolymers using ultrafast RAFT polymerisation, Polym. Chem., 2019, 10, 1186-1191, DOI: 10.1039/C8PY01437J
About the Web writer
 Dr. Athina Anastasaki is an Editorial Board Member and a Web Writer for Polymer Chemistry. Since January 2019, she joined the Materials Department of ETH Zurich as an Assistant Professor to establish her independent research group.
Dr. Athina Anastasaki is an Editorial Board Member and a Web Writer for Polymer Chemistry. Since January 2019, she joined the Materials Department of ETH Zurich as an Assistant Professor to establish her independent research group.