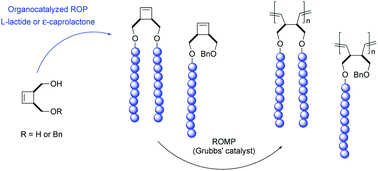
Driven by the interest in developing new efficient methodologies to prepare well-defined grafted poly(1,4-butadiene)s, Fontaine and co-workers used in the present work a consecutive organocatalyzed ring-opening polymerization (ROP)/ROMP route to prepare poly(1,4-butadiene)-g-polyesters from cyclobutenyl macromonomers bearing one or two polyester segment(s) derived from L-lactide (LA) or ε-caprolactone (CL). The products resulting from this strategy represent the first examples of poly(1,4-butadiene)-g-polyesters through the macromonomer route. These results pave the way for more complicated macromolecular architectures, e.g., by modification of the side-chain termini. Moreover, the hydrolytic (bio)degradation potential of the side chains (that can be used as sacrificial domains) of those bottlebrush copolymers makes them attractive candidates to be used for the preparation of complex hollowed nanostructures.
Synthesis and polymerization of cyclobutenyl-functionalized polylactide and polycaprolactone: a consecutive ROP/ROMP route towards poly(1,4-butadiene)-g-polyesters by Flavien Leroux, Véronique Montembault, Sagrario Pascual, William Guerin, Sophie M. Guillaume and Laurent Fontaine Polym. Chem.2014, 5, 3476-3486.
Julien Nicolas is a web-writer and advisory board member for Polymer Chemistry. He currently works at Univ. Paris-Sud (FR) as a CNRS researcher.