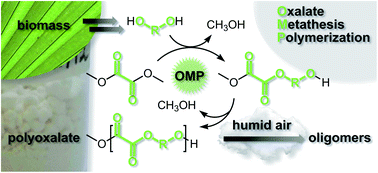
In this study, Garcia and Miller were inspired to develop improved synthetic routes and novel families of polyoxalates, particularly targeting thermal properties that might allow mimicry of popular packaging thermoplastics. Polyalkylene oxalates and polyarylene oxalates were successfully synthesized via oxalate metathesis polymerization (OMP), a step-growth polymerization that employs acid-catalyzed ester interchange of dimethyl oxalate and a diol in a 1:1 ratio. The process was optimized in the melt and did not require solvents. Aliphatic/aromatic polyoxalate copolymers derived from 1,10- decanediol and resorcinol bis(hydroxyethyl)ether or hydroquinone bis(hydroxyethyl)ether in varying compositions were also prepared and studied. Incorporation of the aromatic diols into the polymer chain generally afforded increased Tg and Tm. Solid-state degradation studies indicated facile water-degradation of the polyoxalates as molecular weights decreased 81-92% over the course of 13 months in humid air.
Polyoxalates from biorenewable diols via Oxalate Metathesis Polymerization by John J. Garcia and Stephen A. Miller Polym. Chem. 2014, 5, 955-961.
Julien Nicolas is a web-writer and advisory board member for Polymer Chemistry. He currently works at Univ. Paris-Sud (FR) as a CNRS researcher.