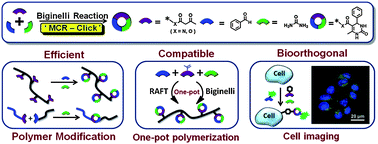
In this context, Tao, Wei and co-workers have revisited the Biginelli reaction as a potential tri-component click chemistry. Through the quick and efficient modification of polymer side groups and locking of two polymer chains, Biginelli-type homopolymers and Biginelli-locked copolymers can be facilely and quantitatively obtained. Moreover, the Biginelli reaction showed good compatibility with RAFT polymerization to construct a one-pot MCP system. Both reactions proceeded well without interference, and almost neat Biginelli functionalized homopolymers were successfully achieved in a one-pot fashion. More importantly, the Biginelli reaction can also be recognized as a ‘catalyst-free’ bioorthogonal-click reaction, through which a fluorescent probe can be covalently anchored onto cell membranes without external addition of a catalyst, implying the potential application of the Biginelli reaction in chemical biology.
A new insight into the Biginelli reaction: the dawn of multicomponent click chemistry? by Chongyu Zhu, Bin Yang, Yuan Zhao, Changkui Fu, Lei Tao and Yen Wei Polym. Chem. 2013, 4, 5395-5400.
Julien Nicolas is a guest web-writer and advisory board member for Polymer Chemistry. He currently works at Univ. Paris-Sud (FR) as a CNRS researcher.