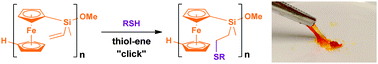Block copolymers are known to self-assemble into micellar structures when placed in a solvent selective for one of the blocks. A range of morphologies have been reported, such as spherical, cylindrical and vesicular architectures. It has been previously shown that cylindrical micelles can be prepared from diblock copolymers containing a short, crystalline polyferrocenyldimethylsilane (PFDMS) block when placed in a solvent selective for the second longer block. Only a few examples of PFS-based cylindrical micelles with a metal-containing corona have been reported so far. Although PFSs with a range of properties have been developed by changing the substituent on silicon, this approach requires the development of a new monomer for each new material.
In this context, Manners and co-workers reported an investigation of the reactions between various monofunctional thiols and vinyl-containing PFS scaffolds as a potential tool for the preparation of functional all-PFS materials. Thiol–ene click functionalization of PFS homopolymers was successfully carried out with a range of thiols, and the resulting materials were found to show interesting features (solubility, thermal transitions). Considering the diversity of available thiols and the great tolerance of the thiol–ene reaction towards functional groups, this post-polymerization route appears to be the candidate of choice for the preparation of PFS materials with a range of properties from the same homopolymer or diblock scaffold, thus avoiding the difficulties associated with the preparation of new monomers.
Controlled thiol–ene post-polymerization reactions on polyferrocenylsilane homopolymers and block copolymers by Laurent Chabanne, Stefan Pfirrmann, David J. Lunn and Ian Manners, Polym. Chem., 2013, 4, 2353-2360.
Julien Nicolas is a guest web-writer for Polymer Chemistry. He currently works at Univ. Paris-Sud (FR) as a CNRS researcher.