Supramolecular polymers, defined as polymeric arrays of many repeating units held together by reversible and weak non-covalent interactions, are considered as the result of the combination of supramolecular chemistry and polymer science. Non-covalent interactions, such as multiple hydrogen bonding, host–guest interactions and metal-coordination, have been introduced to fabricate supramolecular polymers, and these reversible and highly directional secondary interactions endow supramolecular polymers with novel topological structures and unique functions. Charge transfer complexes, prepared by the association between an electron acceptor and an electron donor, have been proved to be important and attractive building blocks for the construction of multiple supramolecular aggregates.
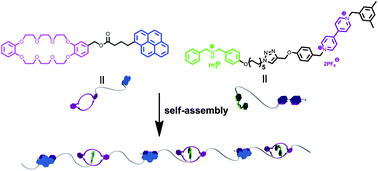
In this view, Huang and co-workers reported on the design and the synthesis of a novel supramolecular polymer constructed from two heteroditopic monomers driven by the combination of crown ether-based and charge-transfer molecular recognition. High molecular weight supramolecular polymers were formed by complexation between crown ethers and secondary ammonium salts, and paraquat derivatives and pyrene derivatives, respectively. This kind of supramolecular polymer exhibited the ability to construct nanofibers via electrospinning technology.
A supramolecular polymer formed by the combination of crown ether-based and charge-transfer molecular recognition by Shengyi Dong, Lingyan Gao, Jianzhuang Chen, Guocan Yu, Bo Zheng and Feihe Huang, Polym. Chem., 2013, 4, 882-886.
Julien Nicolas is a guest web-writer for Polymer Chemistry. He currently works at Univ. Paris-Sud (FR) as a CNRS researcher.