Synthetic polymers have experienced an exponential growth over the last sixty years, in part due to their combined low density and exceptional mechanical properties. Along with chain architecture and microdomain morphology, polymer crystallinity is one of the determining parameters which control these properties. Recently, there has been a flurry of activity regarding the possibility of altering mechanical properties by changing chain architecture or microdomain morphology upon the action of a chemical stimulus, a property which can be exploited for the fabrication of mechanically adaptive objects as well as sensors.
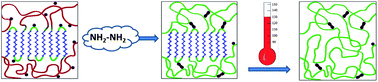
In this context, Claverie and co-workers reported a functional polymer with chemically switchable crystallinity. Linear polyethylenes containing pendant diacetone acrylamide groups were prepared using Pd phosphine sulfonate catalysts. These polymers are easily cross-linked upon reaction with hydrazine, and the cross-links can be cleaved by ozonolysis to regenerate the original polymer. The cross-linked polymer, once heated above the melting point, becomes permanently amorphous, as the crosslinks prevent the chains from packing. Crystallinity can only be recovered with the cleavage of the cross-links. Thus, the polymeric material exhibits two states (crystalline and amorphous) which are triggered upon action of simple chemicals. This study thus offers a proof of principle that crystallinity responds to the action of a chemical stimulus.
A functional polymer with chemically switchable crystallinity by Jean-Christophe Daigle, Alexandre A. Arnold, Laurence Piche and Jerome P. Claverie, Polym. Chem., 2013, 4, 449-452.
Julien Nicolas is a guest web-writer for Polymer Chemistry. He currently works at Univ. Paris-Sud (FR) as a CNRS researcher.