Although the primitive single-cyclic form conceptually represents the simplest cyclic topology, the synthetic constraints in general prevent from the clean and easy production of uniform cyclic polymers, particularly when availability of a specific functional group for further topological upgrade is desired. The common syntheses of single-cyclic polymers employ end-to-end ring-closure and ring-expansion polymerization.
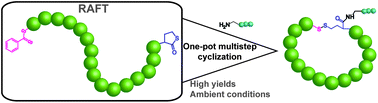
In this study, Du Prez and co-workers reported on an efficient synthetic pathway toward cyclic polymers based on the combination of thiolactone and disulfide chemistry. First, heterotelechelic linear polystyrene (PS) containing an a-thiolactone (TLa) and an w-dithiobenzoate group was synthesized via reversible addition–fragmentation chain transfer (RAFT) polymerization, employing a newly designed TLa-bearing chain transfer agent (CTA). The subsequent reaction of this heterotelechelic polymer with an amine, which acts as a nucleophile for both the TLa and dithiobenzoate units, generated the a,w-thiol-telechelic PS under ambient conditions without the need for any catalyst or other additives. The arrangement of thiols under a high dilution afforded single cyclic PS (c-PS) through an oxidative disulfide linkage. Moreover, the authors demonstrated a controlled ring opening via either disulfide reduction or thiol–disulfide exchange to enable easy and clean topology transformation. Furthermore, to illustrate the broad utility of this synthetic methodology, different amines including functional ones were employed, allowing for the one-step preparation of functionalized cyclic polymers with high yields.
Straightforward synthesis of functionalized cyclic polymers in high yield via RAFT and thiolactone–disulfide chemistry by Milan M. Stamenović, Pieter Espeel, Eisuke Baba, Takuya Yamamoto, Yasuyuki Tezuka and Filip E. Du Prez, Polym. Chem., 2013, 4, 184-193.
Julien Nicolas is a guest web-writer for Polymer Chemistry. He currently works at Univ. Paris-Sud (FR) as a CNRS researcher