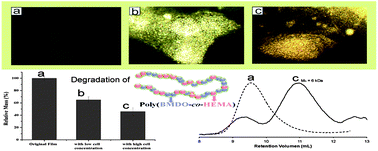
Poly(2-hydroxyethyl methacrylate) (PHEMA) is a widely used and researched biocompatible polymer. However, PHEMA is neither hydrolysable nor enzymatically degradable. Radical-ring-opening copolymerization of cyclic ketene acetals such as 2-methylene-1,3-dioxepane (MDO) and 5,6-benzo-2-methylene-1,3-dioxepane (BMDO) with vinyl monomers has been a promising method for introducing degradable ester linkages onto the C–C polymer backbones. In this article, Agarwal and co-workers reported the synthesis of degradable and less toxic PHEMA with ester linkages in the backbone by radical copolymerization with cyclic ketene acetal. The copolymers were hydrolytically degradable under basic conditions and also showed surface and bulk degradation using macrophages. The authors also demonstrated promising positive results for the use of such polymers as sustained drug delivery systems.
Biocompatible and degradable poly(2-hydroxyethyl methacrylate) based polymers for biomedical applications by Yi Zhang, Dafeng Chu, Mengyao Zheng, Thomas Kissel and Seema Agarwal Polym. Chem. 2012, 3, 2752-2759.
To keep up-to-date with all the latest research, sign up for the journal’s e-alerts or RSS feeds or follow Polymer Chemistry on Twitter or Facebook.