Gold nanostars are gold nanoparticles with multiple branches, a shape which gives rise to their unique properties. These nanoparticles have tuneable localized surface plasmon resonances in the biologically transparent near-IR window, and excitation of these plasmons using a laser creates a local temperature increase. For this reason, gold nanostars have potential for use in non-invasive antitumoral and antibiofilm laser treatments.
The problem faced by scientists, however, is how to achieve a temperature increase that is large enough to be effective, without exposing the overlying skin to a level of irradiation that exceeds the safe limits. This is what Piersandro Pallavicini from the Department of Chemistry at the University of Pavia, and an international team of colleagues, set out to investigate.
They generated gold nanostars with plasmon resonances at 835 and 1530 nm, respectively. Each of these plasmons could be irradiated separately, leading to observable increases in temperature. However, when both plasmons were irradiated simultaneously, the temperature increase was equal to the sum of the temperature increases when the plasmons were irradiated separately.
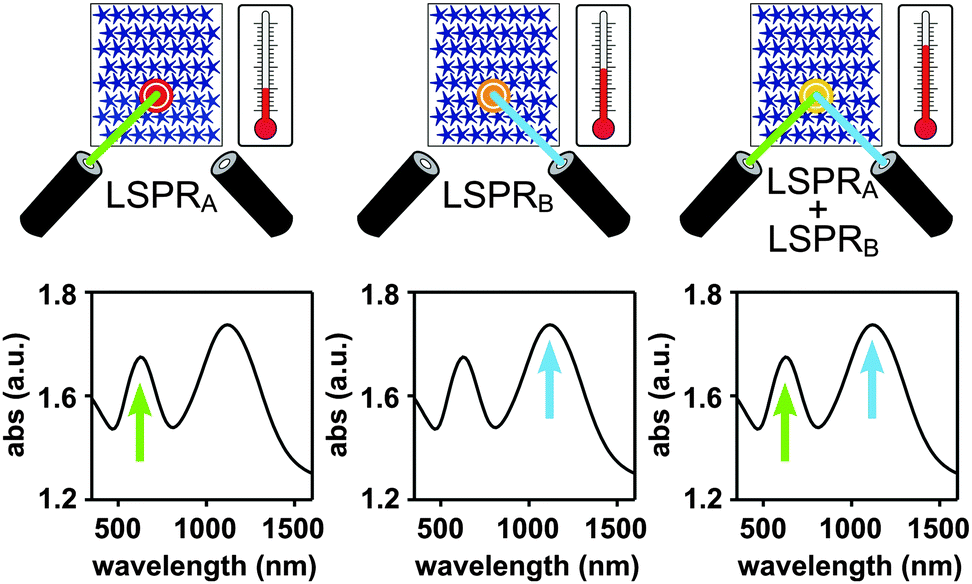
Temperature increases observed from the laser excitation of individual or multiple plasmon resonances of gold nanostars
The implication of these findings is that Pallavicini and colleagues successfully found a way to obtain a larger local temperature increase using irradiation that remains below the safe limits. This significantly increases the potential of gold nanostars for application in the in the treatment of biofilm growth on implants in vivo.
To find out the full details of the additive temperature effect, read the ChemComm article today – it’s free to access until 21st October 2015:
Monolayers of gold nanostars with two near-IR LSPRs capable of additive photothermal response
Piersandro Pallavicini, Simone Basile, Giuseppe Chirico, Giacomo Dacarro, Laura D’Alfonso, Alice Dona, Maddalena Patrini, Andrea Falqui, Laura Sironi and Angelo Taglietti
Chem. Commun., 2015, 51, 12928-12930
DOI: 10.1039/C5CC04144A











 Scientists in Australia have lit the path towards replacing wires in electrochemical devices by
Scientists in Australia have lit the path towards replacing wires in electrochemical devices by  Also of interest:
Also of interest:



![Upon photoexcitation of [Ru(bpy)3]2+, electron transfer through a ferredoxin scaffold to a cobaloxime catalyst facilitates the production of hydrogen.](http://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ImageService/image/GA?id=C5CC03006D) It is the latter approach which
It is the latter approach which 

