The 25 most-downloaded ChemComm articles in the fourth quarter of 2015 were as follows:
Design and synthesis of nitrogen-containing calcined polymer/carbon nanotube hybrids that act as a platinum-free oxygen reduction fuel cell catalyst
Tsuyohiko Fujigaya, Takeshi Uchinoumi, Kenji Kaneko and Naotoshi Nakashima
Chem. Commun., 2011, DOI: 10.1039/C1CC11303H, Communication
Improvement of oxygen reduction reaction and methanol tolerance characteristics for PdCo electrocatalysts by Au alloying and CO treatment
Yu-Chen Wei, Chen-Wei Liu and Kuan-Wen Wang
Chem. Commun., 2011, DOI: 10.1039/C1CC15110J, Communication
Ultrasensitive electrochemical immunoassay of proteins based on in situ duple amplification of gold nanoparticle biolabel signals
Xiaoli Qin, Aigui Xu, Ling Liu, Wenfang Deng, Chao Chen, Yueming Tan, Yingchun Fu, Qingji Xie and Shouzhuo Yao
Chem. Commun., 2015, DOI: 10.1039/C5CC01439E, Communication
Multifunctional catalysis by Pd-polyoxometalate: one-step conversion of acetone to methyl isobutyl ketone
Robert D. Hetterley, Elena F. Kozhevnikova and Ivan V. Kozhevnikov
Chem. Commun., 2006, DOI: 10.1039/B515325E, Communication
The surface chemistry of metal–organic frameworks
Christina V. McGuire and Ross S. Forgan
Chem. Commun., 2015, DOI: 10.1039/C4CC04458D, Feature Article
From themed collection 2015 Emerging Investigators
Production of few-layer phosphorene by liquid exfoliation of black phosphorus
Jack R. Brent, Nicky Savjani, Edward A. Lewis, Sarah J. Haigh, David J. Lewis and Paul O’Brien
Chem. Commun., 2014, DOI: 10.1039/C4CC05752J, Communication
π-Electron rich small molecule sensors for the recognition of nitroaromatics
Sankarasekaran Shanmugaraju and Partha Sarathi Mukherjee
Chem. Commun., 2015, DOI: 10.1039/C5CC07513K, Feature Article
Nanostructured electrochromic smart windows: traditional materials and NIR-selective plasmonic nanocrystals
Evan L. Runnerstrom, Anna Llordés, Sebastien D. Lounis and Delia J. Milliron
Chem. Commun., 2014, DOI: 10.1039/C4CC03109A, Feature Article
Yttrium alkyl complexes with a sterically demanding benzamidinate ligand: synthesis, structure and catalytic ethene polymerisation
Sergio Bambirra, Daan van Leusen, Auke Meetsma, Bart Hessen and Jan H. Teuben
Chem. Commun., 2003, DOI: 10.1039/B208502J, Communication
Graphene quantum dots: emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices
Jianhua Shen, Yihua Zhu, Xiaoling Yang and Chunzhong Li
Chem. Commun., 2012, DOI: 10.1039/C2CC00110A, Feature Article
Reduction of graphene oxide viaL-ascorbic acid
Jiali Zhang, Haijun Yang, Guangxia Shen, Ping Cheng, Jingyan Zhang and Shouwu Guo
Chem. Commun., 2010, DOI: 10.1039/B917705A, Communication
Aggregation-induced emission: phenomenon, mechanism and applications
Yuning Hong, Jacky W. Y. Lam and Ben Zhong Tang
Chem. Commun., 2009, DOI: 10.1039/B904665H, Feature Article
Key processes in ruthenium-catalysed olefin metathesis
David J. Nelson, Simone Manzini, César A. Urbina-Blanco and Steven P. Nolan
Chem. Commun., 2014, DOI: 10.1039/C4CC02515F, Feature Article
Recent developments in transition metal carbides and nitrides as hydrogen evolution electrocatalysts
Wei-Fu Chen, James T. Muckerman and Etsuko Fujita
Chem. Commun., 2013, DOI: 10.1039/C3CC44076A, Feature Article
From themed collection Electrocatalytic hydrogen evolution
A facile synthesis of UiO-66, UiO-67 and their derivatives
Michael J. Katz, Zachary J. Brown, Yamil J. Colón, Paul W. Siu, Karl A. Scheidt, Randall Q. Snurr, Joseph T. Hupp and Omar K. Farha
Chem. Commun., 2013, DOI: 10.1039/C3CC46105J, Communication
Layered double hydroxides toward electrochemical energy storage and conversion: design, synthesis and applications
Mingfei Shao, Ruikang Zhang, Zhenhua Li, Min Wei, David G. Evans and Xue Duan
Chem. Commun., 2015, DOI: 10.1039/C5CC07296D, Feature Article
A superior catalyst with dual redox cycles for the selective reduction of NOx by ammonia
Zhiming Liu, Yang Yi, Junhua Li, Seong Ihl Woo, Baoyi Wang, Xingzhong Cao and Zhuoxin Li
Chem. Commun., 2013, DOI: 10.1039/C3CC43041C, Communication
Rapid synthesis of zeolitic imidazolate framework-8 (ZIF-8) nanocrystals in an aqueous system
Yichang Pan, Yunyang Liu, Gaofeng Zeng, Lan Zhao and Zhiping Lai
Chem. Commun., 2011, DOI: 10.1039/C0CC05002D, Communication
Aerobic oxidation catalysis with stable radicals
Qun Cao, Laura M. Dornan, Luke Rogan, N. Louise Hughes and Mark J. Muldoon
Chem. Commun., 2014, DOI: 10.1039/C3CC47081D, Feature Article
Strongly green-photoluminescent graphene quantum dots for bioimaging applications
Shoujun Zhu, Junhu Zhang, Chunyan Qiao, Shijia Tang, Yunfeng Li, Wenjing Yuan, Bo Li, Lu Tian, Fang Liu, Rui Hu, Hainan Gao, Haotong Wei, Hao Zhang, Hongchen Sun and Bai Yang
Chem. Commun., 2011, DOI: 10.1039/C1CC11122A, Communication
Synthesis and catalytic properties of MIL-100(Fe), an iron(III) carboxylate with large pores
Patricia Horcajada, Suzy Surblé, Christian Serre, Do-Young Hong, You-Kyong Seo, Jong-San Chang, Jean-Marc Grenèche, Irene Margiolaki and Gérard Férey
Chem. Commun., 2007, DOI: 10.1039/B704325B, Communication
Nano-structured ternary niobium titanium nitrides as durable non-carbon supports for oxygen reduction reaction
Minghui Yang, Abigail Rose Van Wassen, Rohiverth Guarecuco, Héctor D. Abruña and Francis J. DiSalvo
Chem. Commun., 2013, DOI: 10.1039/C3CC45732J, Communication
Chemical synthesis of magnetic nanoparticles
Taeghwan Hyeon
Chem. Commun., 2003, DOI: 10.1039/B207789B, Feature Article
Yolk/shell nanoparticles: new platforms for nanoreactors, drug delivery and lithium-ion batteries
Jian Liu, Shi Zhang Qiao, Jun Song Chen, Xiong Wen (David) Lou, Xianran Xing and Gao Qing (Max) Lu
Chem. Commun., 2011, DOI: 10.1039/C1CC13658E, Feature Article
Exploration of the medical periodic table: towards new targets
Nicolas P. E. Barry and Peter J. Sadler
Chem. Commun., 2013, DOI: 10.1039/C3CC41143E, Feature Article
From themed collection Medicinal Inorganic Chemistry
ChemComm is the home of urgent high quality communications from across the chemical sciences. With a world renowned reputation for quality and fast times to publication (average of 40 days), ChemComm is the ideal place to publish your research.
Submit your urgent research to ChemComm today!
Stay up to date with ChemComm
Be among the first to hear about the newest articles being published –
Sign-up to our journal news alert to receive information about most read articles, themed issues, journal news, as well as calls for papers and invitations.
Comments Off on Top 25 ChemComm articles October–December 2015
 Scientists in the UK have developed supramolecular cages that can trap chemical weapon simulants using the hydrophobic effect.
Scientists in the UK have developed supramolecular cages that can trap chemical weapon simulants using the hydrophobic effect.













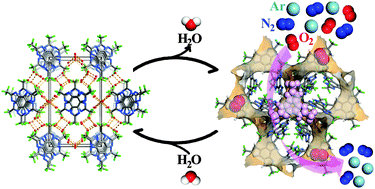

 European scientists have developed an
European scientists have developed an 
