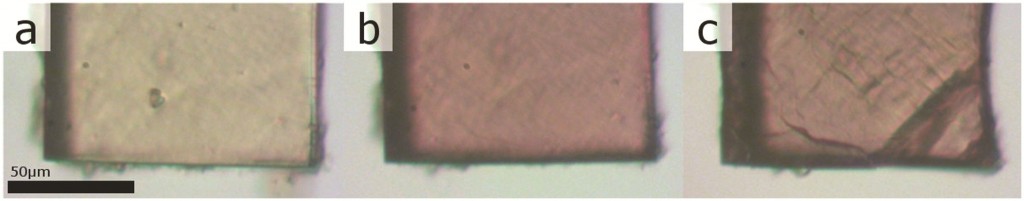
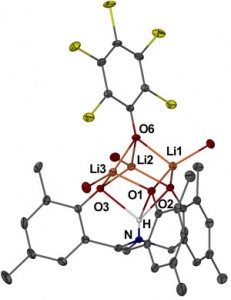
The synthesis and characterization of a new class of photochromic metal-organic framework (MOF) linkers is described in this communication. Dinesh Patel from Pennsylvania State University and collaborators from the Benedict Research Group at University of Buffalo demonstrate that additional functionality, such as photoswitching, can be designed into a ligand without affections the topology of MOFs.
Compounds that change their molecular and electronic structure upon application of light are ideal candidates for sensors, switches and optical data storage medial. These photochromic molecules are now being pursued for use in MOFs in the hope of affording photonic control over the physical properties of the crystalline host. Several instances of MOFs containing non-covalently attached photochromic molecule have been reported, but there is a lack of control over guest orientation and concentration. The use of photoactive linkers means that the photochromic groups are covalently attached to the framework leading to MOFs with well-defined stoichiometry. In this report, a new class of photoswitchable linkers, based on diarylethene photochromes is introduced.
This article has been highlighted as a news story ‘metal organic frameworks react to light’ by Nina Notman in Materials Today
To read more about the full synthesis and characterization, including crystal structure analysis of reaction intermediates, download the full article for free*
Dinesh G. (Dan) Patel, Ian M. Walton, Jordan M. Cox, Cody J. Gleason, David R. Butzer and Jason B. Benedict
DOI: 10.1039/C3CC49666J
*Access is free untill the 19th May 2014 through a registered RSC account – click here to register