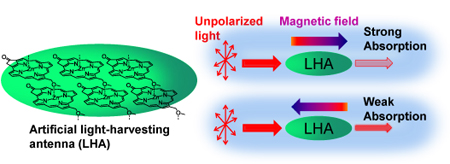
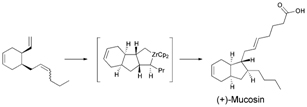
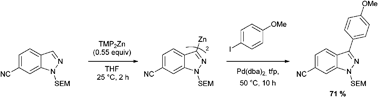
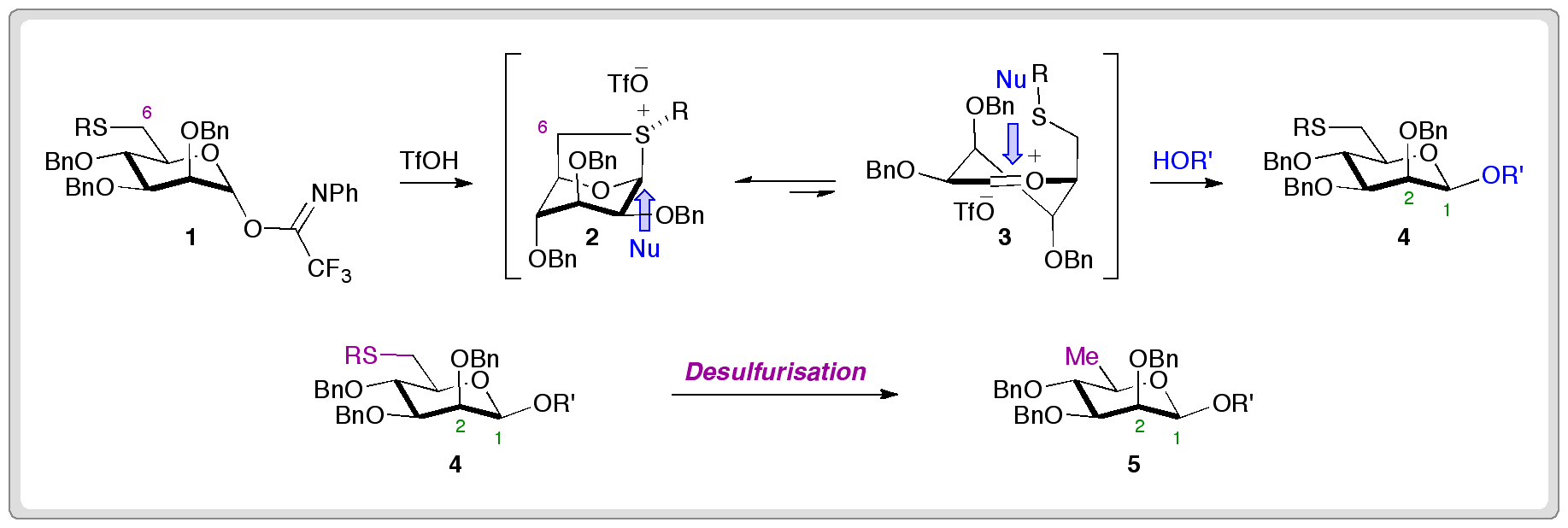
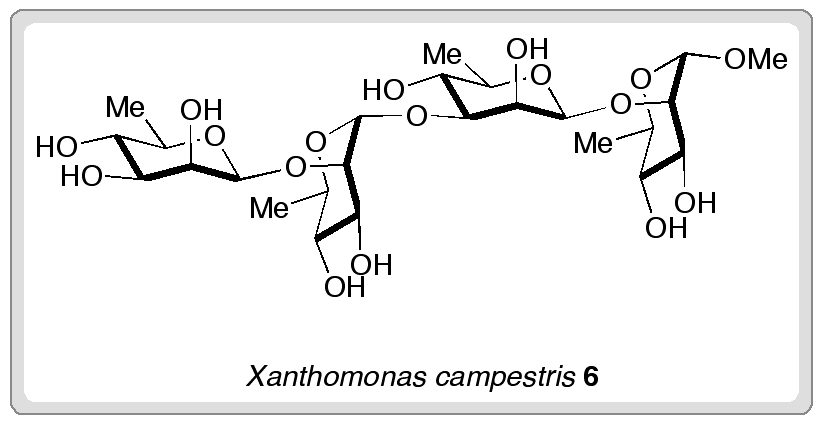
Researchers from the University of Oxford have completed the first synthesis of (±)-hydroxyanthecotulide, an antiparasitic molecule that displays a number of other interesting biological activities.
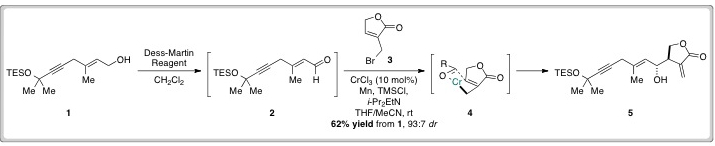
David Hodgson’s group used their previously developed Cr(II)-catalysed allylation reaction to construct the molecule’s carbon skeleton. Alcohol 1 was constructed in a single step and then oxidised to aldehyde 2, which was used crude in an allylation reaction with 3 to give the anti-product (5) in good yields and high levels of diastereoselectivity.
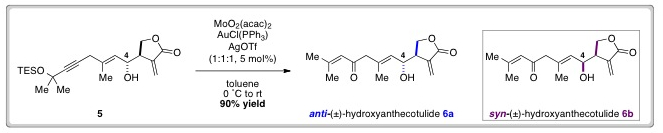
The desired enone functionality was revealed by a Meyer-Schuster rearrangement of 5, which proceeded in excellent yield with in situ desilylation occurring under the reaction conditions.
1H and 13C NMR spectra of 6a were then compared with spectra of an authentic sample of (±)-hydroxyanthecotulide. Discrepancies in this spectral data encouraged the researchers to synthesise syn–6b, by inversion of the C-4 secondary alcohol.
Gratifyingly, the spectra of syn-(±)-hydroxyanthecotulide (6b) was found to match data for the authentic sample and HPLC analysis provided further evidence to confirm that natural (±)-hydroxyanthecotulide, possesses syn-stereochemistry.
This research enabled the synthesis of both anti– and syn-(±)-hydroxyanthecotulide in 5 and 7 steps respectively, and may provide an attractive synthetic route for access to analogues of this biologically relevant family of molecules.