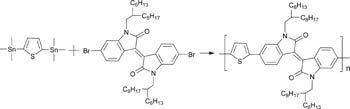
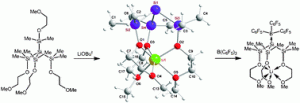
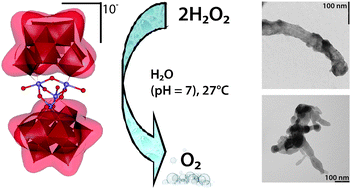
Bioanalysis and biosensors represent a current area of wide interest in the chemical sciences. Much work is being put into methods that allow easy separation and detection of biological targets. Erkang Wang and coworkers at the Chinese Academy of Sciences in Changchun have presented a new way of doing just that.
The group synthesised a new type of silver micro-dendrite (SMD) that has a silver surface perfect for tethering biomolecules. The SMDs could also be reversibly separated and dispersed in water merely by oscillation and then settling under gravity for 30 seconds.
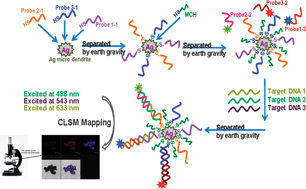
The team used the SMDs to successfully detect DNA from sickle cell disease, human T-lymphotropic virus and anthrax. Probe DNA was attached to the SMDs and then the target strands were selectively bound with a second, fluorophore-containing probe. The target DNA could then be detected using laser scanning confocal microscopy. They found that this technique was so sensitive it could even detect DNA with a one nucleotide mismatch.
Wang hopes that this type of separation system can be expanded to more targets and other types of sensing system.
Interested in finding out more? Then download the ChemComm article for free today and leave a comment below.












 Chinese researchers have shown for the first time that nanomaterials made from titanium dioxide (TiO2) can be used in cigarette filters to significantly reduce the amount of harmful chemicals inhaled by smokers. They say it offers a cheaper and safer alternative than using carbon-based nanomaterials and show potential for use in other filtering devices including gas masks and air purification systems.
Chinese researchers have shown for the first time that nanomaterials made from titanium dioxide (TiO2) can be used in cigarette filters to significantly reduce the amount of harmful chemicals inhaled by smokers. They say it offers a cheaper and safer alternative than using carbon-based nanomaterials and show potential for use in other filtering devices including gas masks and air purification systems.