UK scientists have developed a probe to monitor bicarbonate concentrations in mitochondria – components in living cells that generate chemical energy. Monitoring bicarbonate levels will improve researchers’ understanding of its role in cellular reaction mechanisms.
A challenge when designing cellular probes is ensuring that the probe is not only selective for its target but can also be delivered to the site of interest within the cell. A team of scientists led by David Parker at the University of Durham has made a probe that can overcome this challenge.
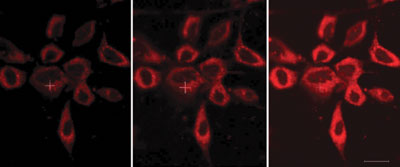 The luminescent probe features an azaxanthone moiety, which is linked to a europium complex by an amide bond. The azaxanthone allows the probe’s uptake into cells and localisation within the mitochondria, and the europium complex has an affinity for bicarbonate ions. The ability to probe bicarbonate levels ‘can offer an unprecedented insight into signalling mechanisms’, says Parker.
The luminescent probe features an azaxanthone moiety, which is linked to a europium complex by an amide bond. The azaxanthone allows the probe’s uptake into cells and localisation within the mitochondria, and the europium complex has an affinity for bicarbonate ions. The ability to probe bicarbonate levels ‘can offer an unprecedented insight into signalling mechanisms’, says Parker.
Read the rest of this story in Chemistry World and download Professor Parker’s ChemComm communication, which is free to access for a limited period.
Also of interest:
Definition of the uptake mechanism and sub-cellular localisation profile of emissive lanthanide complexes as cellular optical probes
Elizabeth J. New, Aileen Congreve and David Parker, Chem. Sci., 2010, 1, 111-118