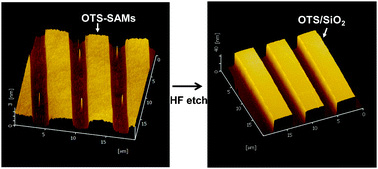
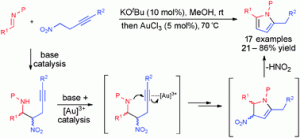
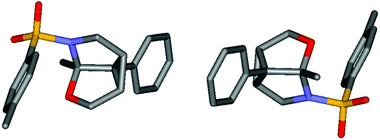Gold-catalysed cycloisomerisation of alkynyl hydroxyallyl tosylamides has led to the concise synthesis of oxaazatricyclic compounds and revealed an interesting skeleton structure, say scientists in South Korea.
Research groups across the globe have reported cycloisomerisation reactions of alkynyl allyl alcohols (AAAs) with rhodium, iridium, palladium and nickel catalysts. Naturally, research groups have been led into investigating the use of gold catalysts, providing plausible reaction pathways for these reactions.
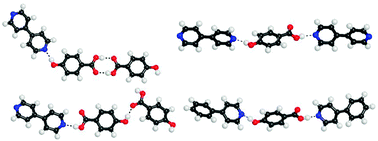
Young Keun Chung and colleagues from Seoul National University and Sangmyung University, used subtly different reaction conditions than have been previously reported, resulting in an usual oxaazatricyclic skeleton compound being uncovered for the first time, as shown in the X-ray structure obtained below.
To find out more about this unexpected result and the reaction conditions used, why not download the communication today? The results have been published in ChemComm and will be freely available until the 13th May 2011!