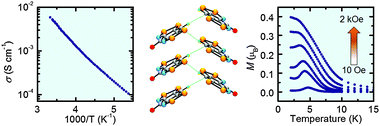
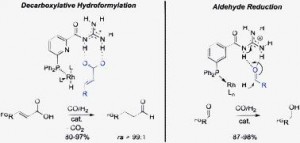
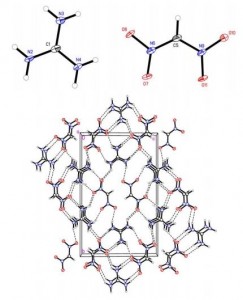
Supramolecular architecture, crystal structure and transport properties of the prototypal oxobenzene-bridged bisdithiazolyl radical conductor
Joanne W. L. Wong, Aaron Mailman, Stephen M. Winter, Craig M. Robertson, Rebecca J. Holmberg, Muralee Murugesu, Paul A. Dube and Richard T. Oakley
Chem. Commun., 2013, Advance Article
DOI: 10.1039/C3CC46686H, Communication
Free to access until 1st December 2013
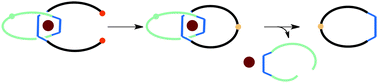
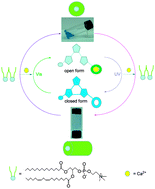
Synthesis of a metal-free coordinating ring via formation of a cleavable [2]catenane
Frédéric Niess and Jean-Pierre Sauvage
Chem. Commun., 2013,49, 10790-10792
DOI: 10.1039/C3CC46452K, Communication
Free to access until 1st December 2013
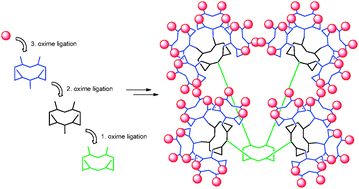
Expanding the scope of oxime ligation: facile synthesis of large cyclopeptide-based glycodendrimers
Baptiste Thomas, Nathalie Berthet, Julian Garcia, Pascal Dumy and Olivier Renaudet
Chem. Commun., 2013,49, 10796-10798
DOI: 10.1039/C3CC45368E, Communication
Free to access until 1st December 2013
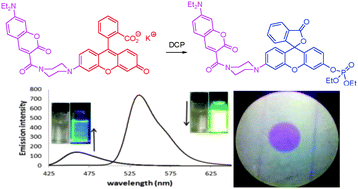
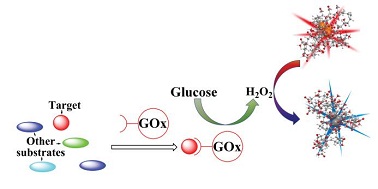
A FRET-based ratiometric fluorescent and colorimetric probe for the facile detection of organophosphonate nerve agent mimic DCP
Weimin Xuan, Yanting Cao, Jiahong Zhou and Wei Wang
Chem. Commun., 2013,49, 10474-10476
DOI: 10.1039/C3CC46095A, Communication
Free to access until 1st December 2013
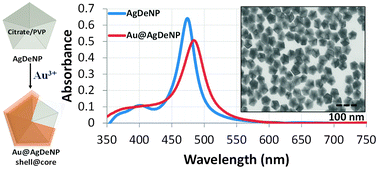
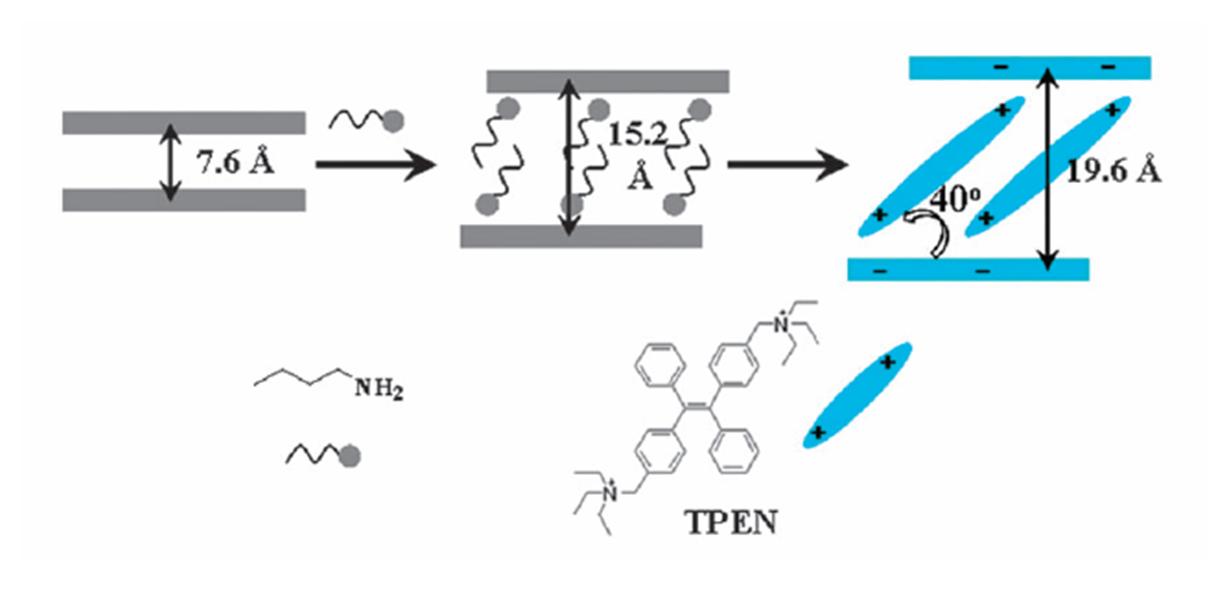
Gold plating of silver nanoparticles for superior stability and preserved plasmonic and sensing properties
Nimer Murshid, Ilya Gourevich, Neil Coombs and Vladimir Kitaev
Chem. Commun., 2013, Advance Article
DOI: 10.1039/C3CC46075D, Communication
Free to access until 1st December 2013
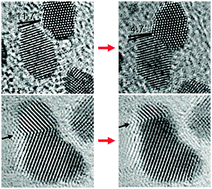
In situ atomic imaging of coalescence of Au nanoparticles on graphene: rotation and grain boundary migration
Jong Min Yuk, Myoungho Jeong, Sang Yun Kim, Hyeon Kook Seo, Jihyun Kim and Jeong Yong Lee
Chem. Commun., 2013, Advance Article
DOI: 10.1039/C3CC46545D, Communication
From themed collection Structure and chemistry of materials from in-situ electron microscopy
Free to access until 1st December 2013