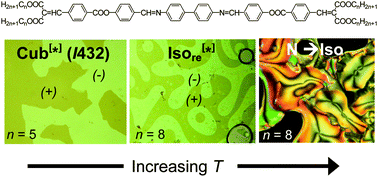
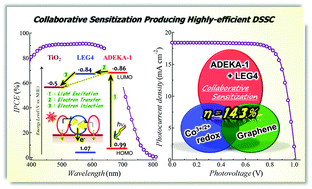
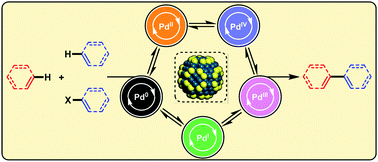
Pharmaceutical cocrystals – cocrystals that contain a drug molecule – can improve the physicochemical performance of drugs, making the prescription you take on doctor’s orders more effective. The properties of any crystal are inherently dependent upon composition and crystal packing, so if you have control over these two things you have control over the physicochemical properties.
Following an explosion of interest and work in this area over the last decade, Michael Zaworotko and colleagues from the Department of Chemical and Environmental Sciences at the University of Limerick review the current state of the literature on pharmaceutical cocrystals.
They cover four areas: nomenclature, design using hydrogen-bonded supramolecular synthons, methods of discovery, and synthesis and development of pharmaceutical cocrystals as drug products. Usefully, they present seven recent case studies on the clinical improvements that can be observed.
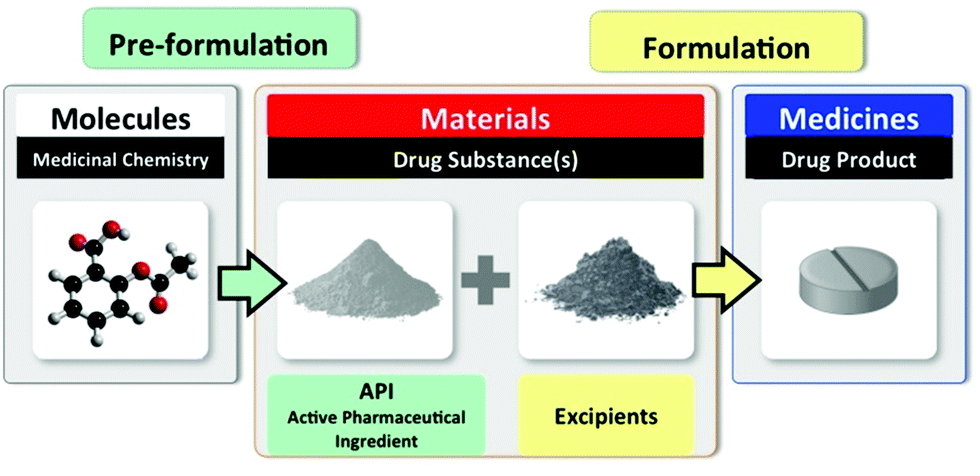
The three stages of early drug discovery and development: identify a molecule that is biologically active; create a material suitable for use in a drug product; formulate the material into a medicine with excipients.
A review in 2004 asked the question “Do pharmaceutical co-crystals represent a new path to improved medicines?” Zaworotko and colleagues, having reviewed the last decade of literature, can answer in the affirmative. To find out why the answer to this question is “yes” read this review today!
To read the details, check out the ChemComm article in full:
Pharmaceutical cocrystals: along the path to improved medicines
Naga Duggirala, Miranda Perry, Örn Almarsson and Michael Zaworotko
Chem. Commun., 2016, 52, Advanced Article
DOI: 10.1039/C5CC08216A













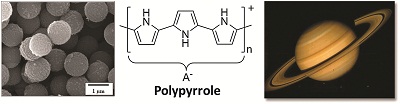
 Scientists in Australia have lit the path towards replacing wires in electrochemical devices by
Scientists in Australia have lit the path towards replacing wires in electrochemical devices by 

![Upon photoexcitation of [Ru(bpy)3]2+, electron transfer through a ferredoxin scaffold to a cobaloxime catalyst facilitates the production of hydrogen.](http://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ImageService/image/GA?id=C5CC03006D) It is the latter approach which
It is the latter approach which 