In 2011, Silvia Marchesan published her first article as a corresponding author in our journal. We wanted to speak to Silvia about why she chose ChemComm as home for Tripeptide self-assembled hydrogels: unexpected twists of chirality.
Read our interview with Silvia here:
What are the main areas of research in your lab and what motivated you to take this direction?
Nature’s choice for homochirality (e.g., L-peptides) has stimulated our research, as we question it with heterochiral molecules. The scientific journey in this direction started from the design of simple and low-cost tripeptides to define self-assembly rules within chemical systems of biological relevance. We typically use 1 or 2 D-amino acids in D,L- tripeptides, and study small libraries with variations in stereochemistry or amino acid sequence. We recently established how chirality affects spatial conformation for assembly from the molecular, through the nano-, micro- and up to the macroscale. In this way, we can link macroscopic properties of the final systems back to the fine structural details of the building blocks. Our systems assemble in benign solvents, such as buffered water or acetonitrile, and design allows the fine-tuning of their lifetime and biodegradation rate. Applications vary, from biomimicry of natural structures to antimicrobial or bioadhesive soft matter (ChemComm 2020), to the bioinspired design of catalysts (ChemComm 2017), whereby function can be switched on/off with assembly/disassembly.
As Alice steps into Wonderland through the mirror, so we like to think that use of the mirror-image of natural L-amino acids enables entry into a supramolecular wonderland, whereby the building blocks are similar overall to their natural counterparts, but with a “magical twist” (indeed, often D-amino acids induce a kink in the backbone). We also like the challenge to combine different systems together at the boundary with nanotechnology: a branch of our research enjoys stimulating collaborations to attain hybrid or composite nanomaterials with carbon nanostructures for new applications in catalysis, biomaterials, biomarker detection, etc.
Can you set this article in a wider context?
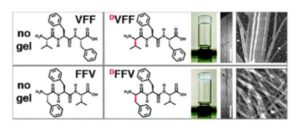
The 2012 Communication set the first example whereby a simple substitution of an L-amino acid with its D-enantiomer in an unprotected (linear) peptide sequence dictated a dramatic change in self-assembly behavior, since the tripeptides with D-L-L stereoconfiguration formed nanostructured hydrogels at physiological conditions, while their homochiral analogues simply precipitated. Moreover, a simple change of order in the amino acid sequence allowed to achieve different nanomorphologies (i.e., twisted fibrils or nanotapes), giving scope for further investigations. It took some years to obtain funding for this research and gather the required resources to identify the rationale behind these observations, as well as to convince skeptics that this approach can indeed be extended to other examples, and can add function to the assemblies. Examples include catalysis (ChemComm 2017) or mimicry of biological structures, such as the extracellular matrix (ECM) to sustain cell culture (ChemComm 2016) or even to induce cell adhesion, with bioactive ECM-derived motifs (ChemComm 2020). The possibility to create a desired function with the assemblies is especially attractive to attain spatiotemporal control over reaction cascades, or to design therapeutics that are activated where and when needed.
What do you hope your lab can achieve in the coming year?
Funding! Our funded projects have ended and further resources are required to make the leap towards tailored applications. These systems have a great potential: research has identified thus far sequences with very interesting properties, such as the ability to inhibit pathological amyloid fibrillization, and to exert antimicrobial activity only when assembled. Manuscripts are in preparation, so… stay tuned! Full-atom molecular dynamics simulations have unveiled how the peptides dance as they assemble, and single-crystal XRD has provided mesmerising photographs of water-channels formed by simple sequences, and with varying diameter in the nanometer scale. Given the required resources, I am confident we can produce useful dynamic systems and perhaps even shed a new light on life’s choice for homochirality.
Describe your journey to becoming an independent researcher.
I fell in love with research at first sight, and the fire burns bright despite the rollercoaster of academic life. My journey was non-conventional, as I simply followed the passion for science that led me from Italy (M.Sc. on fullerenes under the supervision of Profs Prato and Da Ros) to the UK (to work with Dr Macmillan who shared my love for (glyco)protein engineering), Finland (where I joined the group of Prof Gahmberg on integrin biochemistry and protein-protein interactions), Australia (joint postdoc between CSIRO and Monash University to work with Prof Forsythe on nanostructured biomaterials) and then back to Italy. When I was at UCL, after work, I loved to stop by the Wellcome Exhibition Centre and the British Library to get inspired. It is there that I discovered the original drawings of Alice in Wonderland, and I am extremely grateful to my supervisors in Australia for allowing me to explore new research avenues in my “spare” time. I wrote many unsuccessful grants, and after submitting what I thought was going to be the last one (thinking of plan B, out of academia), I hit the jackpot with a starting package from the Italian Ministry of Research (MIUR) through the SIR scheme. That was a game-changer that created momentum, and talented postdocs from abroad were attracted to the team, joining forces to explore the exciting area of supramolecular chemistry.
What is the best piece of advice you have ever been given?
To be true to your dream, and commit to it 100%. Enthusiasm is contagious, and creates very positive dynamics in a team. My PhD supervisor offered plenty of quotes from Star Wars, which in turn I offer to my team now! There is also a mask of Darth Vader next to my desktop, to remind me of the urgent need to prompt and implement positive change, to create a better and more inclusive culture in science.
Why did you choose to publish in ChemComm?
During the PhD I had published one article in ChemComm as first author, and I was impressed by the rapid publication times, simple process, and above all, fair and constructive peer-review comments. The whole experience made me feel welcome and part of the scientific community, reflecting other interactions with the RSC and in the UK. ChemComm offered the perfect platform to publish our proof-of-concept and to sail it out into the wider chemistry community. It was an uplifting and totally unexpected surprise to be sitting on my desk Down Under and receive emails from the other side of the world, from colleagues, and from my PhD supervisor, with congratulations for the work. It is important for emerging PIs to receive support from the community– even a short email made a positive and lasting difference.
 |
 Marchesan’s Group in 2018, before the COVID-19 pandemic |
Silvia moved to UK in 2004 to join Procter & Gamble for an R&D internship, just before taking on a PhD at The University of Edinburgh (UK). She enjoyed also the research environment at UCL (2005-2007), where her PhD supervisor, Dr. Derek Macmillan, had established a new lab. She then moved to the University of Helsinki (2008-2010) as Academy of Finland Fellow, and then to Melbourne as CRSS Fellow (2010-2012) in a joint scheme between Monash University and CSIRO (Australia’s national science agency). In 2015 she set up her independent lab at the University of Trieste (www.marchesanlab.com), and secured a tenure-track position that led her to Associate Professorship (2018) and Habilitation for a Full Professorship (2018). The research potential of heterochiral self-assembling peptides was recognized by Nature Index (2018) and Nature Chemistry (2019). Find the lab on Twitter: @MarchesanLab
Don’t forget to read Silvia’s #ChemComm1st article in our collection ChemComm Milestones – First Independent Articles. Find out more by following the hashtags #ChemComm1st and #ChemCommMilestones on our Twitter.