A fluorescent probe could provide a tool for real-time toxin screening in shellfish and help put an end to seafood related food-poisoning, claim US scientists. Dinoflagellates are organisms commonly found in sea water. Some can be toxic and are associated with harmful algae and bacteria accumulation, which can lead to toxins transferring into shellfish tissue, posing a major threat to food safety.
It is often thought that symbiotic bacteria – bacteria that live or interact with other organisms for a long time – play a key role in the biosynthesis of toxins from dinoflagellates. But this toxin-bacteria interaction has not been confirmed, until now. Michael Burkart and colleagues at the University of California at San Diego have used their findings to develop a fluorescence microscopy tool to screen shellfish for toxin producing dinoflagellates.
Burkart’s team fluorescently-labelled a protein that is taken up by the marine cells responsible for biosynthesising the toxin, okadaic acid. In vivo studies clearly show that the samples producing the toxin glow fluorescent blue under the microscope. The samples that provide a positive response to the probe also show signs of symbiotic bacteria in the cell walls, confirming the toxin-bacteria association.
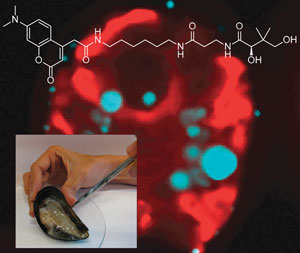
Bacteria in mussels show a blue response under fluorescence
Using this information, Burkhart’s assay is able to select mussels that contain live toxin producing dinoflagellates at different stages of infection by counting the number of cells that fluoresce. Imaging shellfish during dinoflagelate infection detects okadaic acid much quicker than present techniques which can only detect the dinoflagellates once they have been fully absorbed into the shellfish tissue.
Jon Clardy, a pharmacology researcher at the Harvard Medical School in Cambridge, US, says that this work has ‘the beginnings of a potentially useful surveillance tool for public health.’ The main surprise for him was to find out that the bacteria are somehow involved in the biosynthesis of okadaic acid and possibly related to dinoflagellate toxins. This is all the more impressive as Clardy explains, ‘the biosynthesis of these compounds has been untouchable because of the enormous size of dinoflagellate genomes, which are much larger than human genomes, and the lack of genetic systems, which has frustrated commonly used approaches.’
Burkhart says that if this method can be applied to an automated system then it could prove to be a useful screening tool for the aquafarming industry. And looking further to the future he adds, ‘one could imagine a mobile phone application that would let you see if your crop or plate of oysters is safe for consumption. There is a tremendous potential in visual methods for food quality screening and its merge with the modern digital devices and networks.’
Emma Shiells
Fancy reading more? Then why not download the article today and blog your feedback below.
Link to Article
Metabolic probes for imaging endosymbiotic bacteria within toxic dinoflagellates
Carolina P. Reyes, James J. La Clair and Michael D. Burkart, Chem. Commun., 2010
DOI: 10.1039/c0cc02876b