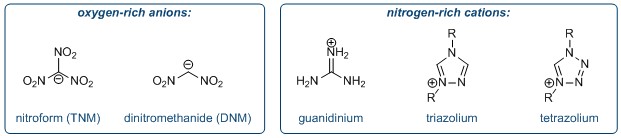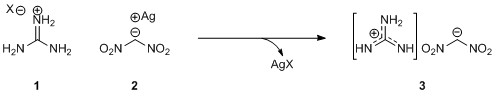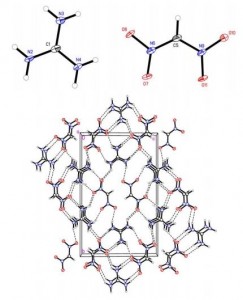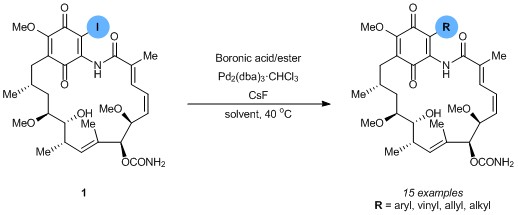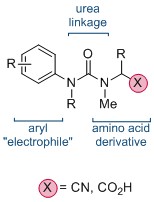
The synthesis of quaternary amino acids is an important challenge facing researchers in bioorganic and medicinal chemistry. While there are a number of ways to transform tertiary amino acids into their quaternary counterparts, α-arylation of amino acids and their derivatives remains limited.
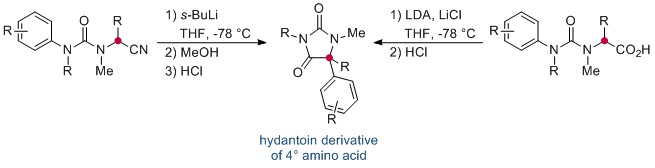
Now, in this HOT ChemComm article, Professor Jonathan Clayden and co-workers at the University of Manchester have revealed an elegant intramolecular arylation of tertiary amino acid derivates, which exploits the use of a urea linkage to connect the amino acid derivative—a nitrile or acid—and the aryl “electrophile”. During the course of the reaction, this N-aryl substituent migrates to the α-carbon of the amino acid moiety. This is followed by a cyclisation, leading to a heterocyclic hydantoin derivative. The reaction is mediated by strong base, and is thought to proceed via the metallated enolate.
Interestingly, the researchers found that the migration of the aryl ring was not influenced by its electronic properties, and that the transition-metal–free reaction could be applied successfully to a range of natural and unnatural tertiary amino acid substrates. If the tertiary amino acid nitrogen is protected with a PMB (p-methoxybenzyl) group, the resulting hydantoin product can subsequently be hydrolysed, affording the acyclic quaternary amino acid.
The reaction was monitored by in situ infrared spectroscopy (ReactIR) to identify the reaction intermediates and cast light on the mechanism of the arylation. Further details of the ReactIR analysis can be found in the electronic supplementary information. Ultimately, Clayden and his group hope to further develop this useful methodology to allow the enantioselective arylation of amino acids.
For more, check out this HOT ChemComm article in full:
Ruth E. Gilligan is a guest web-writer for ChemComm. She has recently completed her PhD in the group of Prof. Matthew J. Gaunt at the University of Cambridge, focusing on the development and application of C–H functionalisation methodology.