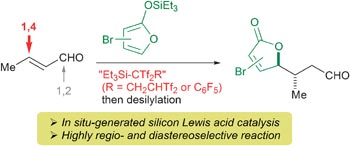
Remarkably different selectivity can be obtained in the reaction of silicon enolates with α,β-unsaturated aldehydes by swapping the classically used Lewis acid catalysts for in situ-generated silicon Lewis acids, say Japanese scientists.
Read more about the conditions used by Takeo Taguchi and colleagues (Tokyo University of Pharmacy and Life Sciences) in their communication, free to access online until 22nd November.