Metal-organic frameworks (MOFs), formed from the assembly of metal ions and organic bridging ligands, often have large pores and high surface areas. Such properties are attractive for various potential applications from gas storage to catalysis. The diverse structures and properties of MOFs can be extended by incorportaing functional groups onto the organic linker after MOF formation through post-synthetic modification.
MIL-101(Cr), a MOF first reported by Ferey et al. in 2005, exhibits mesoporous cages with accessible metal sites as well as high chemical and hydrothermal stability. Amine functionalisation of the framework’s benzene dicarboxylate ligands has been reported for analagous iron and aluminium frameworks, but has so far proven elusive for MIL-101(Cr).
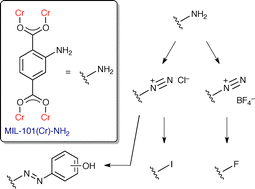 Burrows et al. from the University of Bath have synthesised MIL-101(Cr)-NH2 using a hydrothermal method, and found that the resultant framework is stable up to 250 °C and, most interestingly, is stable to acids. This unusual stability has allowed them to post-synthetically transform the amine group into an arenediazonium salt which they used in situ to generate a variety of functional groups. MIL-101(Cr)-azo, in particular, showed excellent CO2 selectivity at low pressure.
Burrows et al. from the University of Bath have synthesised MIL-101(Cr)-NH2 using a hydrothermal method, and found that the resultant framework is stable up to 250 °C and, most interestingly, is stable to acids. This unusual stability has allowed them to post-synthetically transform the amine group into an arenediazonium salt which they used in situ to generate a variety of functional groups. MIL-101(Cr)-azo, in particular, showed excellent CO2 selectivity at low pressure.
This new approach to post-synthetic modification has provided a flexible route to functionalised MIL-101 materials, with further studies concentrating on selected frameworks already underway.
Read this HOT Chem Comm article today (free to access until the 7th of December 2012):
Synthesis and post-synthetic modification of MIL-101(Cr-NH2) via a tandem diazotisation process
Dongmei Jiang, Luke L. Keenan, Andrew D. Burrows and Karen J. Edler
Chem. Commun., 2012, Advance Article
DOI: 10.1039/c2cc36344e










