The reaction vessel is a fixed variable behind every innovative chemical synthesis, material or catalyst. It may be as simple as a round bottom flask or as complex as a single cell, as large as an industrial batch reactor or as small as a test tube.
Yujun Feng and co-workers, at Sichuan University in China, study a different kind of reaction vessel: water droplets. The droplets are ‘liquid marbles’, composed of microlitre volumes of water with fine hydrophobic particles covering their surface. Liquid marbles can be used as reaction vessels to manipulate small liquid volumes, avoiding the use of specialised microfluidics equipment. In this communication the authors show that carbon dioxide can trigger coalescence of droplets containing multiple reagents, in order to perform microscale chemistry. This research could be useful for developing high-throughput assays for procedures that would benefit from remotely controlled induction such as very fast or hazardous reactions.
The authors synthesised CO2-responsive particles composed of a mixture of polystyrene and PDEA: a methacrylate polymer bearing tertiary amine ancillary groups. The amine is vital to the properties of the polymer: when deprotonated the powder is hydrophobic, but exposure to carbon dioxide renders the polymer hydrophilic by transforming the amine into an ammonium bicarbonate salt. Liquid marbles were synthesised with a patch of CO2-responsive polymer powder. The rest of the marble was coated in lycopodium, a moss spore with hydrophobic properties that is not CO2-responsive (trivia: the high fat content of lycopdoium makes it a great flash powder, used by magicians since the middle ages).
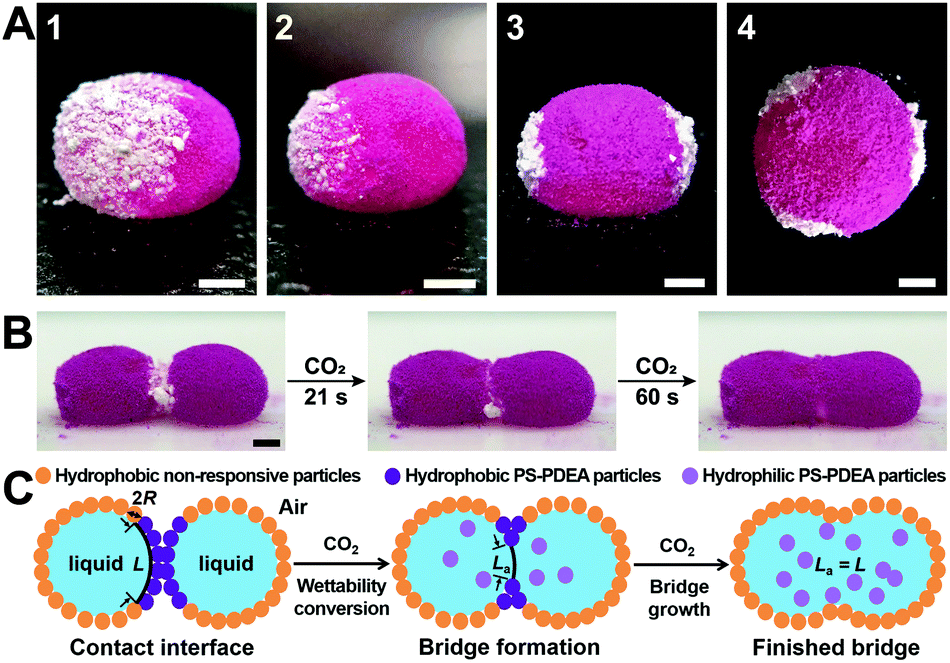
A) Liquid marbles with white CO2-responsive patches and pink (dyed) lycopodium powder. B) & C) Photos and schematic of coalescence between two liquid marbles upon CO2 exposure
To realise CO2-induced chemistry, two liquid marbles containing different chemical reagents are placed side by side with the CO2-responsive powder positioned at the interface of the two marbles. Upon exposure to CO2 the responsive powder becomes hydrophilic and disperses into the aqueous solution within the two marbles, causing them to coalesce and the reagents to react within a single vessel. The authors performed several reactions using this method, all with distinct colour changes for rapid analysis: redox (persulfate and iodide, permanganate and persulfate), complexation (starch and iodine), substitution (bromine water and phenol) and chemiluminescence (luminol, peroxide and ferricyanide).
The authors show in this paper that innovations in chemistry needn’t be limited to reactions themselves; the vessel we choose can broaden what is possible on a practical level. On a completely impractical note, remotely controlled microreactions in liquid marbles sounds like a magic trick, resonant with the lycopodium flash powder covering their surface.
To find out more please read:
CO2-triggered microreactions in liquid marbles
Xinjie Luo, Hongyao Yin, Xian’e Li, Xin Su, Yujun Feng.
Chem. Commun., 2018, Advance Article
DOI: 10.1039/c8cc01786g

About the author
Zoë Hearne is a PhD candidate in chemistry at McGill University in Montréal, Canada, under the supervision of Professor Chao-Jun Li. She hails from Canberra, Australia, where she completed her undergraduate degree. Her current research focuses on transition metal catalysis to effect novel transformations, and out of the lab she is an enthusiastic chemistry tutor and science communicator.










