N-heterocyclic carbenes (NHCs) are an interesting example of chemical curiosity turned commonplace. NHCs are stable singlet carbenes located within an N-heterocycle, in which the carbon centre bears an sp2 hybridised pair of electrons. As early as 1835 chemists were thinking about carbenes, with Dumas’ optimistic (if unsuccessful) attempt to synthesise the methylene carbene by dehydrating methanol. For many years the intentional study of carbenes was considered too demanding because of their instability, and so they remained in relative obscurity. A number of seminal papers changed this preconception; in particular, a report by Wanzlick in 1968 reporting the synthesis of the first NHC-metal complex using mercury and the first synthesis of a stable and isolable NHC by Arduengo in 1991.
Intensification in research and interest in NHCs over the past thirty years may have originated with these seminal reports, but it continues because of the success of NHCs in catalysis: both as strongly σ-donating metal ligands and nucleophilic organocatalysts. One of the most valuable features of NHCs is the ability to tailor their steric and electronic properties by altering the heterocyclic ring and N-bound substituents. Accordingly, the study of NHC reactivity and the development of methods to functionalise NHCs are essential for continued innovation in this field.
Drs Marina Uzelac and Eva Hevia at the University of Strathclyde, Scotland, have written a review article summarising organometallic methods to metallate N-heterocyclic carbenes. The work summarises metallation of all three components of the NHC: the carbenic carbon, the heterocyclic backbone and the N-bound substituents.
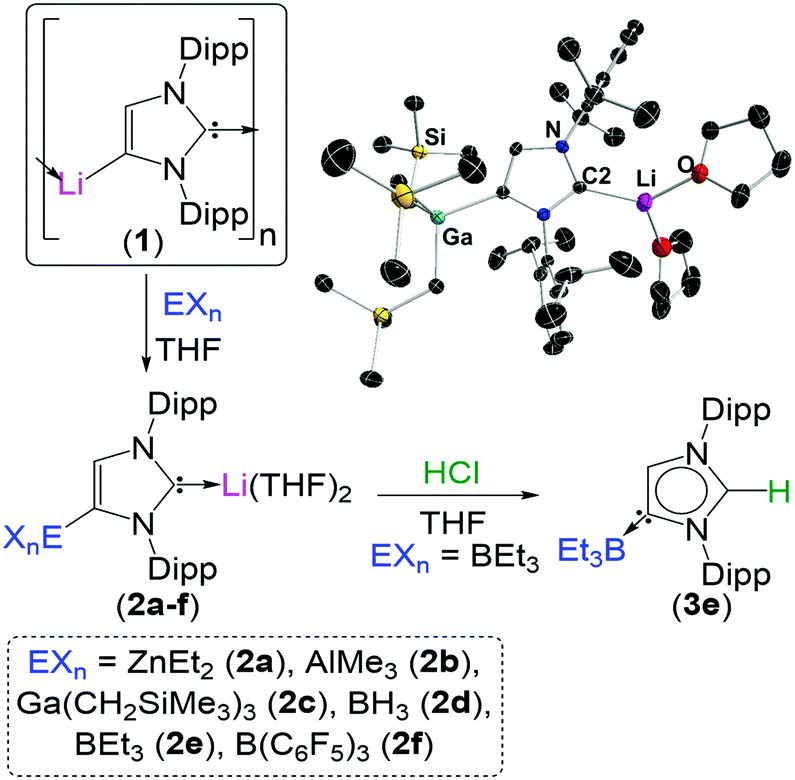
The lithiated complex (1) can be transmetallated at the C4 position by a number of main group elements to give a variety of bimetallic complexes (2). These complexes can be selectively quenched to generate NHC complexes with unconventional regiochemistry (3).
To exemplify the breadth of research discussed; beginning with 2,6-diisopropylphenyl (dipp) substituted imidazole-2-ylidenes, the reactivity of the NHC can be unlocked by initial addition of an alkali metal such as lithium, sodium or potassium (see figure). Metallation at the C4 position occurs by deprotonation of the vinyl protons in the NHC backbone, while a second metal coordinates to the carbene electron pair at the C2 position. From this species (1) it is possible to transmetallate the C4 position with a less-polar metal such as zinc, aluminium, gallium, boron or iron to furnish a bi-metallic NHC (2). Interestingly, addition of an electrophilic methyl or proton source to this species exclusively quenches the C2 position, generating a suite of unconventional complexes (3) with the carbene electron pair positioned on the C4 carbon.
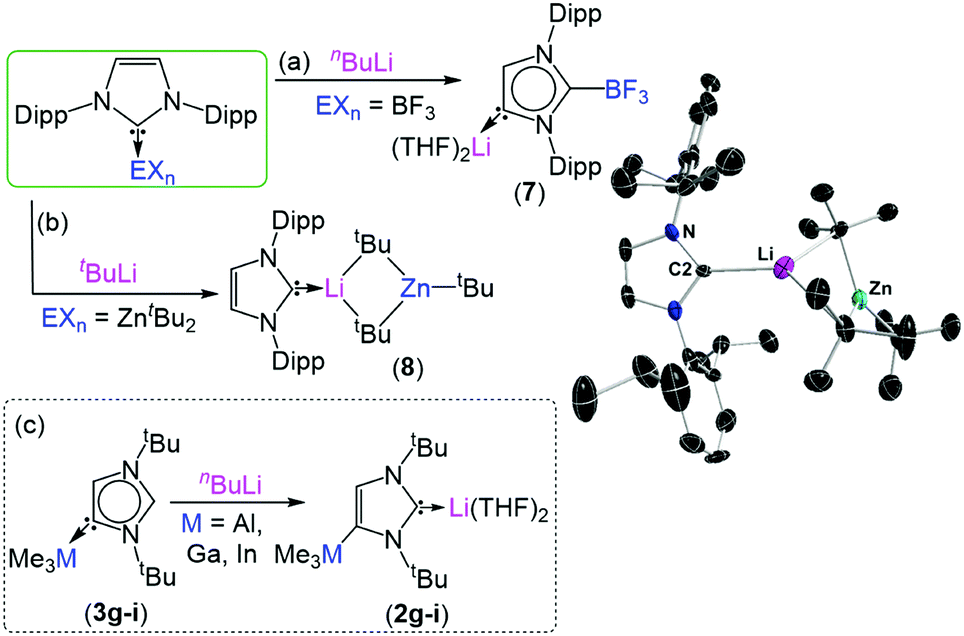
Reactivity of main-group NHC complexes towards lithiation.
Further studies investigate how different reagents influence the regioselectively and extent of metallation, how metallated NHCs can activate small-molecules such as carbon dioxide, conditions which can lead to the metallation of N-dipp substitutents, as well as products and speciation following treatment of NHCs with a variety of bimetallic reagents.
In addition to expanding the knowledge of NHC reactivity, the work summarised in this review provides a reference and inspiration to researchers seeking to tailor NHCs for unique applications in synthesis and catalysis.
To find out more please read:
Polar organometallic strategies for regioselective C-H metallation of N-heterocyclic carbenes
Marina Uzelac and Eva Hevia.
Chem. Commun., 2018, Advance Article
DOI: 10.1039/c8cc00049b
Zoë Hearne is a PhD candidate in chemistry at McGill University in Montréal, Canada, under the supervision of Professor Chao-Jun Li. She hails from Canberra, Australia, where she completed her undergraduate degree. Her current research focuses on transition metal catalysis to effect novel transformations, and out of the lab she is an enthusiastic chemistry tutor and science communicator.











