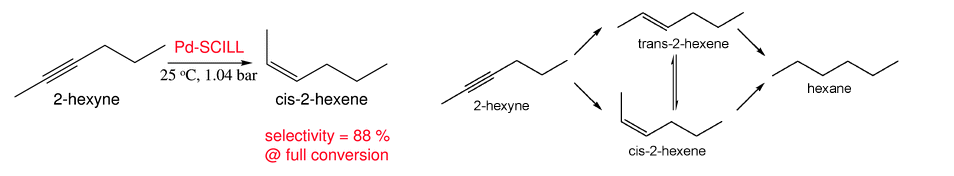
In this ChemComm communication, Peter Claus and co-workers describe an interesting application of room temperature ionic liquids to the selective hydrogenation of 2-hexyne. Unlike many reports in the literature, where an ionic liquid acting as a solvent may enhance a particular reaction, this report outlines a solid supported catalyst system modified with an ionic liquid layer.
Such materials, known as SCILLS, (solid catalyst with an ionic liquid layer) have been investigated in a variety of hydrogenation reactions. In this work the desired reaction is the reduction of 2-hexyne to cis-2-hexene. The catalyst is 1 wt% palladium on silica, modified with various loadings of 3 common ionic liquids: BMIM hexafluorophosphate, BMIM bis(triflouoromethanesulfonyl)imide and N-butyl-N-methylpyrrolidinium dicyanamide ([BMPL][DCA]). The performance of the unmodified catalyst was compared with the yield and selectivity afforded by the SCILL systems. The best results were reported with the dicyanamide ionic liquid SCILL, ([BMPL][DCA]) at 30 wt% ionic liquid loading.
In such a process, there are several reactions that must be suppressed. As the product is an olefin, isomerisation to the trans product must be controlled, as must further hydrogenation to the fully reduced material, hexane. For a number of reasons, based on the nature and amount of chemisorbed hydrogen, and favourable dicyanamide anion interactions with palladium, the dicyanamide SCILL system is particularly effective.
Notably, this system gives improved performance in terms of selectivity and yield over the two best performing commercial catalysts for this task. For example, Lindlar´s catalyst, palladium on calcium carbonate, deactivated with lead, cannot match its performance. In this work, the authors give an example of how ionic liquids can add value to a commercial process, while also offering considerable process improvements, in terms of toxicity and arguably, simplicity. The group’s focus now turns to SCILL activity and stability in a continuous hydrogenation process.
Read this RSC Chemical Communication today!
Frederick Schwab, Natascha Weidler, Martin Lucas and Peter Claus